ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization - PubMed (original) (raw)
ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization
Wenxiang Sun et al. Proc Natl Acad Sci U S A. 2009.
Abstract
We report here the identification and characterization of a protein, ERIS, an endoplasmic reticulum (ER) IFN stimulator, which is a strong type I IFN stimulator and plays a pivotal role in response to both non-self-cytosolic RNA and dsDNA. ERIS (also known as STING or MITA) resided exclusively on ER membrane. The ER retention/retrieval sequence RIR was found to be critical to retain the protein on ER membrane and to maintain its integrity. ERIS was dimerized on innate immune challenges. Coumermycin-induced ERIS dimerization led to strong and fast IFN induction, suggesting that dimerization of ERIS was critical for self-activation and subsequent downstream signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
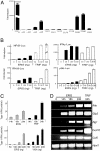
ERIS is a strong type I IFN activator. (A) 293 cells were transiently transfected with 100 ng of pMX-ERIS, along with 50 ng of indicated reporter constructs. A luciferase assay was performed after 24 h. Data represent mean ± SD. (B) 293 cells were transfected with indicated amounts of HA-ERIS or HA-TRIF, along with indicated reporters. A luciferase assay was carried out the same as in A. (C) 293 cells were transfected with 200 ng (Upper) or different dosages (Lower) of 3′HA-ERIS or TRIF/VISA as indicated. Supernatant was collected at different time points (Upper) or at 24 h (Lower) to analyze the concentration of type I IFN production by a type I IFN bioassay. (D) 293 cells were transfected with HA-ERIS or HA-TRIF. Total RNA was prepared at the indicated times after transfection and was analyzed for expression of the indicated genes by RT-PCR. Similar results were obtained in 3 independent experiments.
Fig. 2.
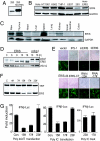
ERIS is involved in innate immunity. (A) 293 cells or 293 cells stably expressing HA-ERIS were lysed and immunoblotted with antibody against ERIS. Immunoblotting analysis was performed on several cell lines (B) or tissues derived from mouse (C) with ERIS antibody. (D) 293 cells were transfected with HA-ERIS. At indicated time points after transfection, cells were infected by VSV for 18 h and then analyzed by immunoblotting with anti-HA or anti-VSV-G. HA-pcbp1 served as a negative control. (E) 293 cells were transfected with empty vector or expression plasmids. Twenty-four or 48 h (for RNAi transfections) later, cells were infected by NDV-GFP (at a MOI of 0.1 or 0.05 for RNAi-transfected cells) for 48 h and imaged by microscopy. (F) 293 cells were transfected with HA-ERIS or HA-TRIF, along with indicated RNAi plasmids. Forty-eight hours later, cells were analyzed by immunoblotting with anti-HA. (G) 293 cells were transfected with a control or ERIS RNAi plasmid (15# or 17#) or with a nonspecific RNAi plasmid (23#), along with 50 ng of IFN-β–Luc reporter plasmid. Twenty-four hours after transfection, 1 μg/mL poly(dA:dT) (Left) or poly(I:C) (Center) was transfected for 18 h, or cells were treated with 200 μg/mL poly(I:C) for 5 h (Right). Cells were analyzed by a reporter assay. Data represent mean ± SD. Similar results were obtained in 3 independent experiments.
Fig. 3.
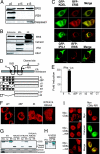
ERIS protein localized to ER membrane and ER retention signal are critical for localization. (A) HT1080 cells (1 × 107) were homogenized in an isotonic buffer and separated into pellet (P) by centrifugation at 5,000 × g (p5), and the supernatant was further centrifuged at 15,000 × g to get p15 and supernatant S15. Different fractions were analyzed with antibody against ERIS, VISA, and caspase-3. (B) HT1080 cells were homogenized and then fractionated by discontinuous sucrose gradient ultracentrifugation. Purified fractions were analyzed with antibody against calnexin (ER), IPS-1/VISA (mitochondrial membrane), and EEA1 (endosome). (C) HeLa cells were cotransfected with red fluorescent protein (RFP)-ERIS and GFP-KDEL (rows 1, 2, and 3) or GFP-VISA (row 4). Cells were fixed and then imaged by fluorescence microscopy (rows 1 and 2) or confocal microscopy (rows 3 and 4). (D) Diagram of ERIS putative ER retention/retrieval sequences and deletions and truncations. Signal peptide (SP): 1–18aa, TM1: 21–37aa, TM2: 48–67aa, TM3: 112–135aa, TM4: 158–175aa, RYR: 76–78aa, RIR: 178–180aa. (E) One hundred nanograms of indicated ERIS mutants was transiently transfected into 293 cells, along with 50 ng of IFN-β–Luc plasmid. Twenty-four hours later, a reporter assay was performed. (F) HeLa cells were transfected with indicated plasmids and then fixed and imaged by confocal microscopy. (G) 293 cells transfected with full-length ERIS or indicated ERIS mutants were subjected to fraction isolation. Fractions were analyzed with antibodies against ERIS, calnexin, or caspase-3. Cyt, cytosol; WCL, whole-cell lysate. (H) The same as G except that the reverted ERIS (AYA/AIA to RYR/RIR) was transfected into 293 cells and the localization of ERIS was analyzed by Western blotting. (I) HeLa cells were transfected with Myc-CD8α, Myc-CD8α-RIR, or Myc-CD8α-AIA. Transfected cells were labeled with antibody against Myc (Left) or were transfected with Myc-CD8α-RIR and labeled with the indicated antibodies (Right). All images are representative of at least 3 independent experiments. (Scale bar: 10 μm.)
Fig. 4.
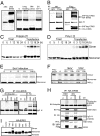
ERIS protein is modified on overexpression or innate immune challenge. (A) 293 cells (293) or 293 cells transfected with HA-ERIS (293-ERIS; Right) were lysed and immunoblotted with anti-HA. (Right) Cell lysates were heated with SDS buffer containing 200 μM DTT for 10 min (0) or 30 min (prolonged heating), heated with SDS buffer containing 400 μM DTT (2× DTT), or heated after addition of 8 M urea into lysis buffer (8 M urea). (B) 293 cells were cotransfected with 3× Flag-ERIS and HA-ERIS. After 24 h, cell lysates underwent immunoprecipitation (IP) with anti-Flag, followed by immunoblotting (IB) with anti-HA and then anti-Flag. 293 cells stably expressing TLR3 were transfected with HA-ERIS. Thirty-six hours later, cells were treated or transfected with poly(dA:dT) (C) or poly(I:C) (D) for indicated times. Cells were lysed and immunoblotted with anti-HA. (E) 293 cells were transfected with HA-ERIS; 36 h later, cells were infected with Sendai virus (SeV) for indicated times, lysed, and immunoblotted with anti-HA. (F) HT1080 cells were infected with SeV or VSV for indicated times, and ERIS dimerization was detected by native gel electrophoresis using antibody against ERIS. (G and H) 293 cells were transfected with HA-ERIS together with empty vector (vector) or Flag-tagged expression plasmids as indicated. After 24 h, cell lysates underwent IP with anti-HA, followed by IB with indicated antibodies. (H, Lower) The whole-cell lysate (WCL) was immunoblotted with anti-HA and anti-Flag antibodies together. (I) 293 cells were transfected with HA-ERIS together with empty vector (control) or Flag-tagged expression plasmids as indicated. After 24 h, cell lysates were directly separated and immunoblotted with anti-HA and anti-Flag antibodies.
Fig. 5.
ERIS dimerization is mediated by its TM domains and leads to type I IFN induction. (A) Diagram of ERIS deletions and truncations used. (B) Indicated expression plasmids (F, Flag-tagged; H, HA-tagged) were cotransfected into 293 cells. Twenty-four hours later, cell lysates underwent immunoprecipitation (IP) with anti-Flag, followed by immunoblotting with anti-HA and then anti-Flag (Upper), or whole-cell lysate (WCL) was immunoblotted with anti-HA and then anti-Flag (Lower). *, Flag-tagged protein; filled triangle, HA-tagged protein. (C) 293 cells were transiently transfected with ERIS-GyrB. After 24 h, coumermycin was added into cell culture medium for the indicated times (Left) or at increasing concentrations (Right). A luciferase assay was performed. Similar results were obtained in 3 independent experiments. Cell lysates were also separated by a native gel, and the coumermycin-induced ERIS-GyrB dimerization was analyzed by the anti-ERIS antibody (Lower).
Similar articles
- TMED2 Potentiates Cellular IFN Responses to DNA Viruses by Reinforcing MITA Dimerization and Facilitating Its Trafficking.
Sun MS, Zhang J, Jiang LQ, Pan YX, Tan JY, Yu F, Guo L, Yin L, Shen C, Shu HB, Liu Y. Sun MS, et al. Cell Rep. 2018 Dec 11;25(11):3086-3098.e3. doi: 10.1016/j.celrep.2018.11.048. Cell Rep. 2018. PMID: 30540941 - The emerging roles of the STING adaptor protein in immunity and diseases.
Liu X, Wang C. Liu X, et al. Immunology. 2016 Mar;147(3):285-91. doi: 10.1111/imm.12561. Epub 2015 Dec 27. Immunology. 2016. PMID: 26643733 Free PMC article. Review. - MITA/STING-mediated antiviral immunity and autoimmunity: the evolution, mechanism, and intervention.
Zhong B, Shu HB. Zhong B, et al. Curr Opin Immunol. 2022 Oct;78:102248. doi: 10.1016/j.coi.2022.102248. Epub 2022 Sep 30. Curr Opin Immunol. 2022. PMID: 36193584 Review. - Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response.
Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S. Saitoh T, et al. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20842-6. doi: 10.1073/pnas.0911267106. Epub 2009 Nov 19. Proc Natl Acad Sci U S A. 2009. PMID: 19926846 Free PMC article. - STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling.
Ishikawa H, Barber GN. Ishikawa H, et al. Nature. 2008 Oct 2;455(7213):674-8. doi: 10.1038/nature07317. Epub 2008 Aug 24. Nature. 2008. PMID: 18724357 Free PMC article.
Cited by
- ZC3HAV1 facilitates STING activation and enhances inflammation.
Qin D, Song H, Wang C, Ma X, Fu Y, Zhao C, Zhao W, Zhang L, Zhang W. Qin D, et al. Commun Biol. 2024 Oct 30;7(1):1418. doi: 10.1038/s42003-024-07116-2. Commun Biol. 2024. PMID: 39478149 Free PMC article. - TMEM173 is a biomarker of predicting prognosis, immune responses and therapeutic effect in human lung adenocarcinoma.
Zhou P, Wang P, Li B. Zhou P, et al. Discov Oncol. 2024 Oct 30;15(1):604. doi: 10.1007/s12672-024-01482-3. Discov Oncol. 2024. PMID: 39476162 Free PMC article. - Advances in the prerequisite and consequence of STING downstream signalosomes.
Lu X, Li X, Li L, Han C, Li S. Lu X, et al. Med Rev (2021). 2024 May 8;4(5):435-451. doi: 10.1515/mr-2024-0016. eCollection 2024 Oct. Med Rev (2021). 2024. PMID: 39444795 Free PMC article. Review. - A TBK1-independent primordial function of STING in lysosomal biogenesis.
Lv B, Dion WA, Yang H, Xun J, Kim DH, Zhu B, Tan JX. Lv B, et al. Mol Cell. 2024 Oct 17;84(20):3979-3996.e9. doi: 10.1016/j.molcel.2024.08.026. Epub 2024 Sep 19. Mol Cell. 2024. PMID: 39423796 - The role of cGAS-STING signaling in rheumatoid arthritis: from pathogenesis to therapeutic targets.
Zhu Q, Zhou H. Zhu Q, et al. Front Immunol. 2024 Sep 25;15:1466023. doi: 10.3389/fimmu.2024.1466023. eCollection 2024. Front Immunol. 2024. PMID: 39386207 Free PMC article. Review.
References
- Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. - PubMed
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. - PubMed
- Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. - PubMed
- Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials