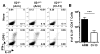A vital role for interleukin-21 in the control of a chronic viral infection - PubMed (original) (raw)
A vital role for interleukin-21 in the control of a chronic viral infection
John S Yi et al. Science. 2009.
Abstract
Understanding the factors that regulate the induction, quality, and longevity of antiviral T cell responses is essential for devising rational strategies to prevent or combat infections. In this study, we show that interleukin-21 (IL-21), likely produced by CD4+ T cells, directly influences the generation of polyfunctional CD8+ T cells and that the number of CD4+ T cells that produce IL-21 differs markedly between acute and chronic infections. IL-21 regulates the development of CD8+ T cell exhaustion and the ability to contain chronic lymphocytic choriomeningitis virus infection. Thus, IL-21 serves as a critical helper factor that shapes the functional quality of antiviral CD8+ T cells and is required for viral control.
Figures
Fig. 1
Diminished IL-21+ CD4+ T cell responses during the initial phase of LCMV-Cl 13 infection. IL-21 and IFN-γ production by LCMV GP61-80 CD4+ T cells was determined eight days following LCMV-Arm or Cl 13 infections of B6 mice. (A) Flow cytometric analysis of intracellular staining for IL-21 and IFN-γ in splenocytes from LCMV infected _Il21_−/− and Il21+/+ mice after stimulation with GP61-80 peptide. Gated total CD4+ T cells are shown. (B) Enumeration of IL-21-producing CD4+ T cells at eight days after LCMV-Arm or Cl 13 infection. Graphs represent mean ± SD; ***P < 0.001. Representative results are shown from two independent experiments (n = 8–9 for Il21+/+ cohorts and n = 2 for _Il21_−/− mice).
Fig. 2
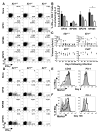
Severe CD8+ T cell exhaustion and viral persistence in the absence of IL-21. Splenic CD8+ T cell responses and viral titers were evaluated following LCMV-Cl 13 infection of Il21+/+, +/−, and −/− mice. (A) Flow cytometric analysis of intracellular cytokine staining for IFN-γ and IL-2 production by CD8+ T cells at eight days following infection after restimulation without or with the indicated peptide epitopes. Gated CD8+ T cells are shown and the percentages of CD8+, IFN-γ+ cells that co-produce IL-2 are reported in parentheses. (B) Percentages of epitope-specific CD8+, IFN-γ+ cells that coproduce IL-2 at eight days following infection. Error bars are SEM; * P<0.05 by comparison with Il21+/+ group. (C) Serum viral titers over time following LCMV-Cl 13 infection of Il21+/+, +/−, −/−, and _Cd4_−/− mice. Results from individual mice are shown; the dotted line represents the limit of detection. (D) IFN-γ and IL-2 production by LCMV-specific CD8+ T cells at 136 days following infection. Gated CD8+ T cells are shown. (E and F) CD43 and PD-1 expression by GP33 tetramer+ CD8+ T cells from Il21+/+ (shaded), +/− (dashed line), and −/− (bold line) mice at eight (E) and 136 days (F) post-infection. The Il21+/− data shown in (D) and (F) are from mice that were aviremic at the time of analysis. Representative or composite data are shown from two independent experiments (n=3–6).
Fig. 3
IL-21 acts directly to sustain virus-specific CD8+ T cells during an ongoing infection. Cohorts of control Il21r+/+/IL21r+/+ (CD45.1/CD45.2) and experimental IL21r+/+/IL21r_−/− (CD45.1/CD45.2) mixed bone-marrow chimeras were infected with LCMV-Cl 13 and CD8+ T cell responses evaluated over time. (A) PBMCs were evaluated by flow cytometry to check reconstitution of CD8+ T cells in Il21r+/+/Il21r_+/+ or Il21r+/+/Il21r_−/− mixed bone-marrow chimeras prior to infection. Gated CD8+ T cells are shown. (B) Flow cytometric analysis of GP33- and GP276-specific CD8+ T cell responses in the circulation at days eight and 16 after infection. Gated tetramer+ CD8+ T cells are shown. (C) Flow cytometric analysis of splenic CD8+ T cells and GP33- and GP276-specific responses at three weeks following infection. Gated CD8+ (left panel) or CD8+ tetramer+ (right panels) cells are shown. (D) Absolute numbers of GP33- and GP276-specific CD8+ T cells in mixed bone-marrow chimeras three weeks following infection. Graphs represent average + SD of Il21r+/+ CD45.1 CD8+ T cells (black), Il21r+/+ CD45.2 CD8+ T cells (gray) and Il21r_−/− CD45.2 CD8 T cells (white). **P < 0.01, ***P < 0.001. Representative results are shown from one of two similar experiments (n= 7 and 8 for the Il21r+/+/IL21r+/+ and IL21r+/+_/IL21r_−/− cohorts, respectively)
Fig. 4
IL-21 treatment enhances CD8+ T cell responses and reduces viral titers in _Cd4_−/− mice. LCMV-Cl 13 infected _Cd4_−/− mice were either left untreated or administered daily doses of 10μg recombinant IL-21 for eight days. At day nine after infection CD8+ T cell responses and viral loads were analyzed. (A) Flow cytometric analysis of intracellular staining of IFN-γ and IL-2 production in splenic virus-specific CD8+ T cells from control or treated cohorts. Gated CD8+ T cells are shown. The mean-fluorescence-intensity (MFI) of IFN-γ producing CD8+ T cells are reported in parentheses. (B) Flow cytometric analysis of GP33 and NP396 tetramer+ CD8+ T cells. Plots show gated CD8+ T cells. (C) Viral titers were assessed in the serum, lungs, and liver of control and IL-21-treated mice. Dotted line indicates the limit of detection (50 pfu/mL) for serum samples. *P < 0.05. Representative results from one of two independent experiments are shown (n= 7 and 6 for control and treated groups, respectively).
Comment in
- Immunology. A chronic need for IL-21.
Johnson LD, Jameson SC. Johnson LD, et al. Science. 2009 Jun 19;324(5934):1525-6. doi: 10.1126/science.1176487. Science. 2009. PMID: 19541985 No abstract available.
Similar articles
- IL-21 is required to control chronic viral infection.
Elsaesser H, Sauer K, Brooks DG. Elsaesser H, et al. Science. 2009 Jun 19;324(5934):1569-72. doi: 10.1126/science.1174182. Epub 2009 May 7. Science. 2009. PMID: 19423777 Free PMC article. - IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection.
Yi JS, Ingram JT, Zajac AJ. Yi JS, et al. J Immunol. 2010 Oct 15;185(8):4835-45. doi: 10.4049/jimmunol.1001032. Epub 2010 Sep 15. J Immunol. 2010. PMID: 20844201 Free PMC article. - Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4⁺ T cell responses and viral control during chronic infection.
Harker JA, Dolgoter A, Zuniga EI. Harker JA, et al. Immunity. 2013 Sep 19;39(3):548-59. doi: 10.1016/j.immuni.2013.08.010. Epub 2013 Aug 29. Immunity. 2013. PMID: 23993651 Free PMC article. - T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus.
Khanolkar A, Fuller MJ, Zajac AJ. Khanolkar A, et al. Immunol Res. 2002;26(1-3):309-21. doi: 10.1385/IR:26:1-3:309. Immunol Res. 2002. PMID: 12403369 Review. - Induction and maintenance of CD8+ T cells specific for persistent viruses.
van Leeuwen EM, ten Berge IJ, van Lier RA. van Leeuwen EM, et al. Adv Exp Med Biol. 2007;590:121-37. doi: 10.1007/978-0-387-34814-8_9. Adv Exp Med Biol. 2007. PMID: 17191382 Review. No abstract available.
Cited by
- Investigating the role for IL-21 in rabies virus vaccine-induced immunity.
Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. Dorfmeier CL, et al. PLoS Negl Trop Dis. 2013;7(3):e2129. doi: 10.1371/journal.pntd.0002129. Epub 2013 Mar 14. PLoS Negl Trop Dis. 2013. PMID: 23516660 Free PMC article. - Clonal lineage tracing reveals mechanisms skewing CD8+ T cell fate decisions in chronic infection.
Kasmani MY, Zander R, Chung HK, Chen Y, Khatun A, Damo M, Topchyan P, Johnson KE, Levashova D, Burns R, Lorenz UM, Tarakanova VL, Joshi NS, Kaech SM, Cui W. Kasmani MY, et al. J Exp Med. 2023 Jan 2;220(1):e20220679. doi: 10.1084/jem.20220679. Epub 2022 Oct 31. J Exp Med. 2023. PMID: 36315049 Free PMC article. - Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria.
Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Basu R, et al. Immunity. 2012 Dec 14;37(6):1061-75. doi: 10.1016/j.immuni.2012.08.024. Epub 2012 Nov 29. Immunity. 2012. PMID: 23200827 Free PMC article. - CD8+ T Cell Responses during HCV Infection and HCC.
Hofmann M, Tauber C, Hensel N, Thimme R. Hofmann M, et al. J Clin Med. 2021 Mar 2;10(5):991. doi: 10.3390/jcm10050991. J Clin Med. 2021. PMID: 33801203 Free PMC article. Review. - HIV-1 and the immune response to TB.
Walker NF, Meintjes G, Wilkinson RJ. Walker NF, et al. Future Virol. 2013 Jan;8(1):57-80. doi: 10.2217/fvl.12.123. Future Virol. 2013. PMID: 23653664 Free PMC article.
References
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Nature. 1993;362:758. - PubMed
- Oxenius A, Zinkernagel RM, Hengartner H. Immunity. 1998;9:449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI067993/AI/NIAID NIH HHS/United States
- R01 AI067993-03/AI/NIAID NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- AI067993/AI/NIAID NIH HHS/United States
- R01 AI049360/AI/NIAID NIH HHS/United States
- AI049360/AI/NIAID NIH HHS/United States
- R01 AI049360-08/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials