Structure and function of Vps15 in the endosomal G protein signaling pathway - PubMed (original) (raw)
Structure and function of Vps15 in the endosomal G protein signaling pathway
Erin J Heenan et al. Biochemistry. 2009.
Abstract
G protein-coupled receptors mediate cellular responses to a wide variety of stimuli, including taste, light, and neurotransmitters. In the yeast Saccharomyces cerevisiae, activation of the pheromone pathway triggers events leading to mating. The view had long been held that the G protein-mediated signal occurs principally at the plasma membrane. Recently, it has been shown that the G protein alpha subunit Gpa1 can promote signaling at endosomes and requires two components of the sole phosphatidylinositol-3-kinase in yeast, Vps15 and Vps34. Vps15 contains multiple WD repeats and also binds to Gpa1 preferentially in the GDP-bound state; these observations led us to hypothesize that Vps15 may function as a G protein beta subunit at the endosome. Here we show an X-ray crystal structure of the Vps15 WD domain that reveals a seven-bladed propeller resembling that of typical Gbeta subunits. We show further that the WD domain is sufficient to bind Gpa1 as well as to Atg14, a potential Ggamma protein that exists in a complex with Vps15. The Vps15 kinase domain together with the intermediate domain (linking the kinase and WD domains) also contributes to Gpa1 binding and is necessary for Vps15 to sustain G protein signaling. These findings reveal that the Vps15 Gbeta-like domain serves as a scaffold to assemble Gpa1 and Atg14, whereas the kinase and intermediate domains are required for proper signaling at the endosome.
Figures
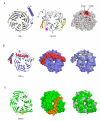
Figure 1. Structure of the Vps15 WD repeat domain and comparison to the beta subunit of transducin (Gβ1)
(A) Left: ribbon diagram of Gβ1. Blades of the propeller are numbered consecutively, as are the strands of one blade. Center: ribbon diagram of Vps15 WD repeat domain, in the same orientation as Gβ1. Breaks within the structure are indicated by dotted lines. The first break occurs at residue 1041; the short stretch of amino acids visible before this break are shown in this part of the figure but deleted in others. Colored regions highlight major differences in the propeller’s structure: blue, N-terminus; magenta, insertion between WD repeats 1 and 2; gold, insertion between WD repeats 2 and 3; green, insertion within WD repeat 6; yellow, insertion within WD repeat 7. The N-terminus and corresponding loop regions of Gβ1 are colored similarly. Right: space filling model of Vps15 WD repeat domain (same orientation) with residues highly conserved across genera colored salmon, and those which are invariant colored red. Arg-1261 and Phe-1262 reside on the surface in the loop between WD repeats 4 and 5. (B) Left: ribbon diagram of Gβγ oriented to highlight the interaction between the beta and gamma (red) subunits. Center: surface representation of Gβγ in the same orientation. Right: surface representation of Gβ1 alone, with hydrophobic residues colored light grey to show the binding cavity for Gγ1. (C) Left: ribbon diagram of Vps15 WD repeat domain oriented as Gβ1 in panel B. The N-terminal loop is colored gold. Center: surface rendering to illustrate partial occlusion of the potential gamma subunit binding surface. Right: surface representation following removal of the N-terminal loop, with hydrophobic residues colored light grey to reveal the absence of a binding cavity for a gamma-like subunit.
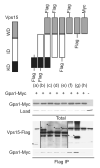
Figure 2. The WD domain of Vps15 is sufficient but not necessary to bind Gpa1
Detergent-solubilized extracts (Total) from cells expressing the indicated Flag fusion proteins and Gpa1 fused to Myc were incubated with Flag resin, eluted with 3X Flag peptide (Flag IP), resolved by 7.5% or 10% SDS-PAGE, and analyzed by immunoblotting with antibodies against Flag, Myc, and G6PDH (Load control). C-terminally tagged forms of (a) and (b) did not express. IP, immunoprecipitation. WD, WD domain. KD, kinase domain. ID, intermediate domain. *, indicates protein of interest.
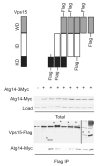
Figure 3. The WD domain of Vps15 is sufficient but not necessary to bind Atg14
Detergent-solubilized extracts (Total) from cells expressing the indicated Flag fusion proteins and Atg14 fused to a triple Myc epitope were incubated with Flag resin, eluted with 3X Flag peptide (Flag IP), resolved by 10% SDS-PAGE, and analyzed by immunoblotting with antibodies against Flag, Myc, and G6PDH (Load control). IP, immunoprecipitation. WD, WD domain. KD, kinase domain. ID, intermediate domain. *, indicates protein of interest.
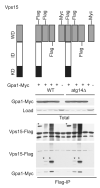
Figure 4. Atg14 is not necessary to mediate the interaction of Vps15 and Gpa1
Detergent-solubilized extracts from wildtype (WT) and _atg14_Δ mutant cells expressing the indicated Flag fusion proteins and Gpa1 fused to a Myc epitope were incubated with Flag resin, eluted with 3X Flag peptide, resolved by 10% SDS-PAGE, and analyzed by immunoblotting with antibodies against Flag, Myc, and G6PDH (Load control). IP, immunoprecipitation. WD, WD domain. KD, kinase domain. ID, intermediate domain. *, indicates protein of interest.
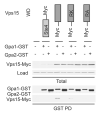
Figure 5. Arg-1261 is necessary for the WD domain of Vps15 to bind efficiently to Gpa1
Detergent-solubilized extracts (Total) from cells expressing the indicated Myc fusion proteins and Gpa1 or Gpa2 fused to GST were incubated with glutathione-Sepharose resin, eluted with glutathione (GST PD, pulldown), resolved by 10% SDS-PAGE, and analyzed by immunoblotting with antibodies against GST, Myc and G6PDH (Load control). PD, pulldown. GST, glutathione S-transferase. WD, WD domain. RA, Arg-1261 to Ala. RK, Arg-1261 to Lys.
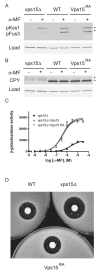
Figure 6. The conserved Arg-1261 is not necessary for G protein signaling at the endosome
(A) Wildtype (WT), _vps15_Δ, and _VPS15_R1261A (Vps15RA) strains were treated with 3μM α-mating factor (MF) for 30 minutes and analyzed by immunoblotting using antibodies against p44/p42 and G6PDH (Load control). (B) The same samples as in panel (A) analyzed using antibodies against carboxypeptidase Y (CPY) and G6PDH. (C) The same strains were transformed with a plasmid containing a pheromone-inducible FUS1-lacZ reporter; transcriptional activation was measured by monitoring β-galactosidase activity in response to pheromone. (D) The same strains were plated and treated with 45 micrograms of α factor to induce cell division arrest. α-MF, alpha mating factor. RA, Arg-1261 to Ala.
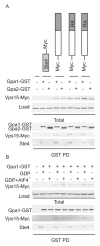
Figure 7. Arg-1261 is not necessary for larger truncations of Vps15 to bind to Gpa1
(A) Detergent-solubilized extracts (Total) from cells expressing the indicated Myc fusion proteins and Gpa1 or Gpa2 fused to GST were incubated with glutathione-Sepharose resin, eluted with glutathione (GST PD), resolved by 10% SDS-PAGE, and analyzed by immunoblotting with antibodies against Myc, GST, Ste4 and G6PDH (Load control). (B) Detergent-solubilized extracts from cells expressing the indicated Myc fusion proteins and Gpa1 fused to GST were lysed in the presence of either GDP or GDP-AlF4-, incubated with glutathione-Sepharose resin, eluted with glutathione, and analyzed by immunoblotting with antibodies against Myc, GST, Ste 4, and G6PDH. PD, pulldown. GST, glutathione S-transferase. RA, Arg-1261 to Ala. RK, Arg-1261 to Lys.
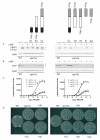
Figure 8. The kinase domain with the intermediate domain of Vps15 is necessary to promote G protein signaling at the endosome
(A) WT and _vps15_Δ cells expressing the indicated Flag fusion proteins or empty vector were treated with 3 μM α-mating factor (MF) for 30 minutes and analyzed by immunoblotting with antibodies against p44/p42 and G6PDH (Load control). (B) The same samples analyzed using antibodies against carboxypeptidase Y (CPY) and G6PDH. (C) The same strains were transformed with a plasmid containing a pheromone-inducible FUS1-lacZ reporter; transcriptional activation was measured by monitoring β-galactosidase activity in response to pheromone. (D) The same strains were plated and treated with 45 micrograms of α factor to induce cell division arrest. α-MF, alpha mating factor.
Similar articles
- Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome.
Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Slessareva JE, et al. Cell. 2006 Jul 14;126(1):191-203. doi: 10.1016/j.cell.2006.04.045. Cell. 2006. PMID: 16839886 - G protein signaling in yeast: new components, new connections, new compartments.
Slessareva JE, Dohlman HG. Slessareva JE, et al. Science. 2006 Dec 1;314(5804):1412-3. doi: 10.1126/science.1134041. Science. 2006. PMID: 17138892 - Regulation of the Saccharomyces cerevisiae Vps34p phosphatidylinositol 3-kinase.
DeWald DB, Wurmser AE, Emr SD. DeWald DB, et al. Biochem Soc Trans. 1997 Nov;25(4):1141-6. doi: 10.1042/bst0251141. Biochem Soc Trans. 1997. PMID: 9449964 Review. No abstract available. - The class III phosphatidylinositol 3-kinase Vps34 in Saccharomyces cerevisiae.
Reidick C, Boutouja F, Platta HW. Reidick C, et al. Biol Chem. 2017 May 1;398(5-6):677-685. doi: 10.1515/hsz-2016-0288. Biol Chem. 2017. PMID: 27935849 Review.
Cited by
- Regulation of the Tumor-Suppressor Function of the Class III Phosphatidylinositol 3-Kinase Complex by Ubiquitin and SUMO.
Reidick C, El Magraoui F, Meyer HE, Stenmark H, Platta HW. Reidick C, et al. Cancers (Basel). 2014 Dec 23;7(1):1-29. doi: 10.3390/cancers7010001. Cancers (Basel). 2014. PMID: 25545884 Free PMC article. Review. - Structural Biology and Electron Microscopy of the Autophagy Molecular Machinery.
Lai LTF, Ye H, Zhang W, Jiang L, Lau WCY. Lai LTF, et al. Cells. 2019 Dec 12;8(12):1627. doi: 10.3390/cells8121627. Cells. 2019. PMID: 31842460 Free PMC article. Review. - Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes.
Rostislavleva K, Soler N, Ohashi Y, Zhang L, Pardon E, Burke JE, Masson GR, Johnson C, Steyaert J, Ktistakis NT, Williams RL. Rostislavleva K, et al. Science. 2015 Oct 9;350(6257):aac7365. doi: 10.1126/science.aac7365. Science. 2015. PMID: 26450213 Free PMC article. - A framework for mapping, visualisation and automatic model creation of signal-transduction networks.
Tiger CF, Krause F, Cedersund G, Palmér R, Klipp E, Hohmann S, Kitano H, Krantz M. Tiger CF, et al. Mol Syst Biol. 2012 Apr 24;8:578. doi: 10.1038/msb.2012.12. Mol Syst Biol. 2012. PMID: 22531118 Free PMC article. - A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi.
Stoetzel C, Bär S, De Craene JO, Scheidecker S, Etard C, Chicher J, Reck JR, Perrault I, Geoffroy V, Chennen K, Strähle U, Hammann P, Friant S, Dollfus H. Stoetzel C, et al. Nat Commun. 2016 Nov 24;7:13586. doi: 10.1038/ncomms13586. Nat Commun. 2016. PMID: 27882921 Free PMC article.
References
- Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. - PubMed
- Dohlman HG, Thorner JW. Regulation of G protein-initiated signal transduction in yeast: Paradigms and principles. Annu. Rev. Biochem. 2001;70:703–754. - PubMed
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. - PubMed
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. - PubMed
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM080739/GM/NIGMS NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- GM081881/GM/NIGMS NIH HHS/United States
- R01 GM081881/GM/NIGMS NIH HHS/United States
- GM080739/GM/NIGMS NIH HHS/United States
- R01 GM073180/GM/NIGMS NIH HHS/United States
- R01 GM080739-03/GM/NIGMS NIH HHS/United States
- R01 GM080739-01/GM/NIGMS NIH HHS/United States
- R01 GM080739-02/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases