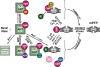Regulation and pharmacology of the mitochondrial permeability transition pore - PubMed (original) (raw)
Review
. 2009 Jul 15;83(2):213-25.
doi: 10.1093/cvr/cvp151. Epub 2009 May 15.
Affiliations
- PMID: 19447775
- PMCID: PMC2701724
- DOI: 10.1093/cvr/cvp151
Review
Regulation and pharmacology of the mitochondrial permeability transition pore
Dmitry B Zorov et al. Cardiovasc Res. 2009.
Abstract
The 'mitochondrial permeability transition', characterized by a sudden induced change of the inner mitochondrial membrane permeability for water as well as for small substances (</=1.5 kDa), has been known for three decades. Research interest in the entity responsible for this phenomenon, the 'mitochondrial permeability transition pore' (mPTP), has dramatically increased after demonstration that it plays a key role in the life and death decision in cells. Therefore, a better understanding of this phenomenon and its regulation by environmental stresses, kinase signalling, and pharmacological intervention is vital. The characterization of the molecular identity of the mPTP will allow identification of possible pharmacological targets and assist in drug design for its precise regulation. However, despite extensive research efforts, at this point the pore-forming core component(s) of the mPTP remain unidentified. Pivotal new genetic evidence has shown that components once believed to be core elements of the mPTP (namely mitochondrial adenine nucleotide translocator and cyclophilin D) are instead only mPTP regulators (or in the case of voltage-dependent anion channels, probably entirely dispensable). This review provides an update on the current state of knowledge regarding the regulation of the mPTP.
Figures
Figure 1
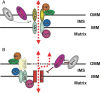
Proposed mPTP complex architecture: (A) Classical view. The mPTP structure is formed by the VDAC–ANT–CyP-D complex, which is located at the ‘contact sites’. Hexokinase II (HKII), mitochondrial creatine kinase (CK), benzodiazepine receptor (PBR), and Bcl-2-family members (Bcl-2, Bcl-xL, and Bax) are included as putative regulatory components. (B) Current view. The core elements comprising the mPTP itself (denoted ‘PTP’ for permeability transition pore) are presently unidentified, but are probably regulated by the adjacent elements as indicated. Note that VDAC, portrayed as a ‘shadow,’ is no longer seen as an essential pore component or even a regulator based on recent genetic evidence. Question mark symbols signify where important open questions remain as far as the participation as a regulator of the mPTP (see text; modified from Juhaszova et al.).
Figure 2
Regulatory roles of ANT, CyP-D, and Pi. The right side of the figure indicates symbolically the threshold for mPTP induction by oxidant stress, whereas the left side indicates mPTP regulatory elements. The middle row (i.e. examined horizontally) indicates the basal state; the regulatory mechanisms shown symbolically in the upper row lead to facilitation of mPTP-induction, while those in the lower row indicate inhibition. Middle row depicts the basal state of ANT and CyP-D as they relate to the basal threshold for mPTP induction by oxidant stress. Top row reflects factors that facilitate mPTP induction: atractyloside, Ca2+, and indirect effects of Pi. Bottom row includes factors that are known to inhibit mPTP induction: genetic deletion of ANT (ANT is dispensable for mPTP formation per se; inhibition of CyP-D by CsA remains protective), ADP or bongkrekic acid (requirement/role of CyP-D under these conditions is unknown), CsA and genetic deletion of CyP-D in the presence of Pi (atractyloside, CsA and Ca2+ are no longer effective when compared with WT). Note the opposing mechanisms of Pi in mPTP-induction: (i) Pi as a direct mPTP desensitizer (bottom row) is opposed by CyP-D binding (top row), whereas (ii) Pi may also act as an indirect mPTP sensitizer (through regulation of Mg2+ and/or polyphosphate levels; top row; see text). Note also that Ca2+ is not a major factor in mPTP induction in cardiomyocytes and neurons. Ppif is the gene encoding CyP-D in mouse.
Figure 3
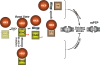
Regulatory roles of VDAC and HKII. Figure layout scheme is the same as in Figure 2. Middle row of the scheme depicts the basal state of VDACs (WT and genetically deleted) and HKII, and their relationship to the basal state and oxidant stress-induced mPTP. mPTP is similar in WT controls as well as in mitochondria lacking VDACs, thus VDAC is dispensable for mPTP-induction. Top row represents facilitation of the ROS-induced mPTP by HKII-VBD peptide: however, because VDAC is dispensable, it is possible that HKII dissociation from some other site may be relevant. Note that this still does not prove that the HKII dissociation from any site by HKII-VBD peptide is casually related to facilitation of mPTP-induction. Bottom row: GSK-3β inhibition results in VDAC dephosphorylation which has been linked with cell protection,, so it remains possible that VDAC phosphorylation may be involved in mPTP regulation.
Figure 4
Proposed model of mPTP modulation by GSK-3β. The phosphorylation state of the mitochondrial-(ANT)-associated pool of GSK-3β contributes to the balance of Bcl-2 family protein effects, the result of which determines the resistance of the mPTP to oxidant stress. Basal state (top): the local GSK-3β pool is active, binds ANT in a complex with phosphorylated VDAC, CyP-D, and possibly other mPTP regulatory elements. ‘Anti-apoptotic’ BH4- and the ‘de-repressor’ BH3-only domain Bcl-2 protein family members are held in mutual check. Protection state (bottom): induced by phosphorylation/inactivation of this mitochondrial GSK-3β subdomain pool resulting in a shift in the balance between Bcl-2 family members toward unmasking the activity of ‘anti-apoptotic’ Bcl-2 family members (and possibly a shift in the interaction between the mPTP and other of its regulators) and consequently in protection of the mPTP against oxidant stress injury (modified from Juhaszova et al.).
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.
Stephenson ZA, Harvey RF, Pryde KR, Mistry S, Hardy RE, Serreli R, Chung I, Allen TE, Stoneley M, MacFarlane M, Fischer PM, Hirst J, Kellam B, Willis AE. Stephenson ZA, et al. Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Pharmacological manipulation of peroxisome proliferator-activated receptor γ (PPARγ) reveals a role for anti-oxidant protection in a model of Parkinson's disease.
Martin HL, Mounsey RB, Mustafa S, Sathe K, Teismann P. Martin HL, et al. Exp Neurol. 2012 Jun;235(2):528-38. doi: 10.1016/j.expneurol.2012.02.017. Epub 2012 Mar 7. Exp Neurol. 2012. PMID: 22417924 Free PMC article. - Mitochondria as a target in treatment.
Frantz MC, Wipf P. Frantz MC, et al. Environ Mol Mutagen. 2010 Jun;51(5):462-75. doi: 10.1002/em.20554. Environ Mol Mutagen. 2010. PMID: 20175113 Free PMC article. Review. - Oxidative Stress Related to Plasmalemmal and Mitochondrial Phosphate Transporters in Vascular Calcification.
Nguyen NT, Nguyen TT, Park KS. Nguyen NT, et al. Antioxidants (Basel). 2022 Mar 2;11(3):494. doi: 10.3390/antiox11030494. Antioxidants (Basel). 2022. PMID: 35326144 Free PMC article. Review. - Leptin Modifies the Rat Heart Performance Associated with Mitochondrial Dysfunction Independently of Its Prohypertrophic Effects.
Berzabá-Evoli E, Zazueta C, Cruz Hernández JH, Gómez-Crisóstomo NP, Juárez-Rojop IE, De la Cruz-Hernández EN, Martínez-Abundis E. Berzabá-Evoli E, et al. Int J Endocrinol. 2018 Aug 1;2018:6081415. doi: 10.1155/2018/6081415. eCollection 2018. Int J Endocrinol. 2018. PMID: 30154842 Free PMC article. - Methyl jasmonate: putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis.
Cesari IM, Carvalho E, Figueiredo Rodrigues M, Mendonça Bdos S, Amôedo ND, Rumjanek FD. Cesari IM, et al. Int J Cell Biol. 2014;2014:572097. doi: 10.1155/2014/572097. Epub 2014 Feb 6. Int J Cell Biol. 2014. PMID: 24648844 Free PMC article. Review.
References
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. - PubMed
- Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. - PubMed
- Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. - PubMed
- Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. - PubMed
- Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991;176:1183–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources