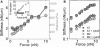Filamin A is essential for active cell stiffening but not passive stiffening under external force - PubMed (original) (raw)
Filamin A is essential for active cell stiffening but not passive stiffening under external force
K E Kasza et al. Biophys J. 2009.
Abstract
The material properties of a cell determine how mechanical forces are transmitted through and sensed by that cell. Some types of cells stiffen passively under large external forces, but they can also alter their own stiffness in response to the local mechanical environment or biochemical cues. Here we show that the actin-binding protein filamin A is essential for the active stiffening of cells plated on collagen-coated substrates. This appears to be due to a diminished capability to build up large internal contractile stresses in the absence of filamin A. To show this, we compare the material properties and contractility of two human melanoma cell lines that differ in filamin A expression. The filamin A-deficient M2 cells are softer than the filamin A-replete A7 cells, and exert much smaller contractile stresses on the substratum, even though the M2 cells have similar levels of phosphorylated myosin II light chain and only somewhat diminished adhesion strength. In contrast to A7 cells, the stiffness and contractility of M2 cells are insensitive to either myosin-inhibiting drugs or the stiffness of the substratum. Surprisingly, however, filamin A is not required for passive stiffening under large external forces.
Figures
Figure 1
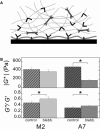
Viscoelastic material properties of M2 and A7 melanoma cells cultured on rigid glass substrates. (A) Elastic, _G_′(ω) (closed), and viscous, _G_″(ω) (open), moduli for A7 (squares) and M2 (circles) cells cultured for 24 h, as measured by MTC. (B) Confocal images of actin cytoskeleton at the basal surface of cells fixed at various times after plating. Bar = 10 _μ_m. (C) Cell stiffness (geometric mean ± SE) measured at ω = 5 rad/s as a function of time after plating; similar time evolution observed for all ω (top). Ratio of viscous to elastic moduli (mean ± SE) of cells as a function of time after plating (bottom). Error bars, if not visible, are smaller than the points in this plot.
Figure 2
Contractile stresses exerted by M2 and A7 cells on collagen I-coated E = 1.3 kPa polyacrylamide substrates. (A) Mean contractile moment M. Examples of contractile deformations induced in the substrate by typical M2 (B) and A7 (C) cells. Image size, 100 _μ_m.
Figure 3
Contractility, stiffness, and spreading of M2 and A7 cells grown on collagen I-coated polyacrylamide substrates of increasing stiffness. (A) Cell contractile moment (mean ± SE). (B) Cell stiffness (geometric mean ± SE). (C) Projected cell area (mean ± SE). (D–I) Examples of cells grown on E = 0.1, 1.3, and 24 kPa substrates.
Figure 4
(A) Schematic showing various roles for filamin A in cell mechanics. Filamin A cross-links actin filaments into orthogonal networks, which support contractile stresses generated by myosin II, and also binds some integrins, linking the actin cytoskeleton to the cell membrane. (B) Effect of myosin II inhibition on material properties of A7 and M2 cells. Cell stiffness (geometric mean ± SE) and the ratio of viscous to elastic moduli (mean ± SE) for control cells and cells treated with 50 _μ_M blebbistatin for 20 min. ∗ indicates that p < 0.05.
Figure 5
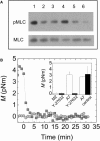
(A) Western blots showing similar levels of pMLC and MLC in M2 (lanes 1–3) and A7 (lanes 4–6) cells plated on collagen I. pMLC levels are decreased in a dose-dependent manner by treatment with 0 _μ_M (lanes 1 and 4), 10 _μ_M (lanes 2 and 5), or 50 _μ_M (lanes 3 and 6) of the Rho-associated kinase inhibitor Y-27632. (B) Contractile moment, M, of a single M2 (circles) or A7 (squares) cell after addition of 10 _μ_M Y-27632 to the media at t = 0. (Inset) Contractile moment (mean ± SE) for cells before (white) and after (black) 20 min of treatment with either 10 _μ_M Y-27632 or control media.
Figure 6
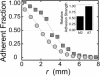
Adhesion strength of M2 (circles) and A7 (squares) cells to collagen I-coated substrates, measured by the spinning disk method (34). After cells are exposed to fluid shear stress, adherent fraction is measured as a function of distance r from the center of the coverslip. Shear stresses on apical surface of cells increase linearly with r. (Inset) Relative adhesion strength determined from radius at which 50% of cells have detached.
Figure 7
Stiffening of A7 and M2 cells subjected to large nanonewton-scale external forces, applied by magnetic tweezers through a fibronectin-coated magnetic bead. (A) Cell stiffness and estimated modulus (geometric mean ± SE) as a function of force for A7 (squares, n = 30) and M2 (circles, n = 19) cells. (Inset) Percentage of beads disrupted from cells as a function of force. (B) Cell stiffness as a function of force for A7 (n = 9) and M2 (n = 7) cells treated with 5 _μ_M cytochalasin-D for 15 min.
Similar articles
- Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin.
Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Byfield FJ, et al. Biophys J. 2009 Jun 17;96(12):5095-102. doi: 10.1016/j.bpj.2009.03.046. Biophys J. 2009. PMID: 19527669 Free PMC article. - An active biopolymer network controlled by molecular motors.
Koenderink GH, Dogic Z, Nakamura F, Bendix PM, MacKintosh FC, Hartwig JH, Stossel TP, Weitz DA. Koenderink GH, et al. Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15192-7. doi: 10.1073/pnas.0903974106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667200 Free PMC article. - Mechanical response of single filamin A (ABP-280) molecules and its role in the actin cytoskeleton.
Yamazaki M, Furuike S, Ito T. Yamazaki M, et al. J Muscle Res Cell Motil. 2002;23(5-6):525-34. doi: 10.1023/a:1023418725001. J Muscle Res Cell Motil. 2002. PMID: 12785102 Review. - Interaction of p38 and Sp1 in a mechanical force-induced, beta 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A.
D'Addario M, Arora PD, Ellen RP, McCulloch CA. D'Addario M, et al. J Biol Chem. 2002 Dec 6;277(49):47541-50. doi: 10.1074/jbc.M207681200. Epub 2002 Sep 24. J Biol Chem. 2002. PMID: 12324467 - Structural and functional aspects of filamins.
van der Flier A, Sonnenberg A. van der Flier A, et al. Biochim Biophys Acta. 2001 Apr 23;1538(2-3):99-117. doi: 10.1016/s0167-4889(01)00072-6. Biochim Biophys Acta. 2001. PMID: 11336782 Review.
Cited by
- Filamin A-hinge region 1-EGFP: a novel tool for tracking the cellular functions of filamin A in real-time.
Planagumà J, Minsaas L, Pons M, Myhren L, Garrido G, Aragay AM. Planagumà J, et al. PLoS One. 2012;7(8):e40864. doi: 10.1371/journal.pone.0040864. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870205 Free PMC article. - Cytoskeletal prestress: The cellular hallmark in mechanobiology and mechanomedicine.
Chowdhury F, Huang B, Wang N. Chowdhury F, et al. Cytoskeleton (Hoboken). 2021 Jun;78(6):249-276. doi: 10.1002/cm.21658. Epub 2021 May 1. Cytoskeleton (Hoboken). 2021. PMID: 33754478 Free PMC article. Review. - Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer.
Tello-Lafoz M, Srpan K, Sanchez EE, Hu J, Remsik J, Romin Y, Calò A, Hoen D, Bhanot U, Morris L, Boire A, Hsu KC, Massagué J, Huse M, Er EE. Tello-Lafoz M, et al. Immunity. 2021 May 11;54(5):1037-1054.e7. doi: 10.1016/j.immuni.2021.02.020. Epub 2021 Mar 22. Immunity. 2021. PMID: 33756102 Free PMC article. - Upregulation of paxillin and focal adhesion signaling follows Dystroglycan Complex deletions and promotes a hypertensive state of differentiation.
Sen S, Tewari M, Zajac A, Barton E, Sweeney HL, Discher DE. Sen S, et al. Eur J Cell Biol. 2011 Feb-Mar;90(2-3):249-60. doi: 10.1016/j.ejcb.2010.06.005. Eur J Cell Biol. 2011. PMID: 20663583 Free PMC article. - A viscoelastic-stochastic model of the effects of cytoskeleton remodelling on cell adhesion.
Li L, Zhang W, Wang J. Li L, et al. R Soc Open Sci. 2016 Oct 19;3(10):160539. doi: 10.1098/rsos.160539. eCollection 2016 Oct. R Soc Open Sci. 2016. PMID: 27853571 Free PMC article.
References
- Fabry B., Maksym G.N., Butler J.P., Glogauer M., Navajas D. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001;87:148102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources