The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection - PubMed (original) (raw)
The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection
Ilona Hauber et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Peptide fragments, derived from prostatic acidic phosphatase, are secreted in large amounts into human semen and form amyloid fibrils. These fibrillar structures, termed semen-derived enhancer of virus infection (SEVI), capture HIV virions and direct them to target cells. Thus, SEVI appears to be an important infectivity factor of HIV during sexual transmission. Here, we are able to demonstrate that epigallocatechin-3-gallate (EGCG), the major active constituent of green tea, targets SEVI for degradation. Furthermore, it is shown that EGCG inhibits SEVI activity and abrogates semen-mediated enhancement of HIV-1 infection in the absence of cellular toxicity. Therefore, EGCG appears to be a promising supplement to antiretroviral microbicides to reduce sexual transmission of HIV-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
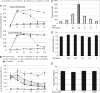
EGCG targets synthetic PAP248–286-derived amyloid fibrils. (A) Amyloid fibrils were formed by agitation of the fresh PAP248–286 solutions (1 and 5 mg/mL) at 37 °C. Fibrillar aggregates were exposed to various concentrations of EGCG (□, 20 mM; ●, 10 mM; ■, 5 mM; and ▲, 1 mM) and detected by Congo red staining at the indicated time points. Addition of PBS alone (solvent for EGCG) served as negative control (×). (B) Preformed PAP248–286-derived fibrils (SEVI) were treated with increasing concentrations of EGCG (▲, 1 mM; △, 2 mM; □, 4 mM; ○, 8 mM; and ●, 10 mM) for 48 h and analyzed as before. Reactions containing EGCG alone (■, 10 mM) or PBS alone (×) served as controls. (C) HIV-1 particles (X4 strain NL4/3) were preincubated for 20 min with or without the indicated concentrations of SEVI. Subsequently, Jurkat 1G5 luciferase indicator cells were exposed to the respective virus/SEVI mixtures for 5 h. At 24 h after infection, RLU/s was determined. Error bars represent three independent experiments. (D) Cellular metabolic activity was tested in uninfected Jurkat 1G5 cells by alamarBlue assay after 5 h of exposure to the indicated concentrations of SEVI. (E) Viabilities of uninfected Jurkat 1G5 cells were determined after 5 h exposure to the indicated EGCG concentrations as before.
Fig. 2.
Transmission electron microscopy analysis of EGCG-treated SEVI in a closed system. Corresponding images are arranged side by side. (A) Dissolved EGCG is shown inside the ultrathin sectioned microtubes and at different magnifications. The overview shows the interface between the tube wall (arrow) and the tube's lumen filled with EGCG. (Magnification: 3000×.) EGCG aggregates are organized in different patterns. (Magnifications: 35,000× and 75,000×.) (B) The SEVI solution shows a high density of SEVI-specific amyloid fibrils. (Magnification: 3,000×.) Because of the embedding angle, the fibrils often run out of the section plane. (Magnification: 28,000×.) Nevertheless, their length differs between 30 and 90 nm. (C) Appearance of SEVI after 12 h of incubation in EGCG. The small EGCG aggregates diffused from the outside through the porous wall into the lumen of the tube covering the surface of the fibrils. (Magnification: 6,300×.) (D) Degradation of the majority of SEVI after 60 h of incubation in 10 mM EGCG.
Fig. 3.
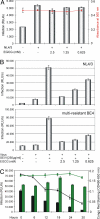
The green tea catechin EGCG inhibits SEVI-mediated HIV infection. (A) Jurkat 1G5 cells were infected with HIV-1 NL4/3 in presence of the indicated concentrations of EGCG. The rate of infection was monitored by measuring the luciferase activity in the respective cell cultures (indicated as RLU/s; bars), and cell viabilities were determined by alamarBlue assay (red plot) 24 h after infection. (B) HIV-1 NL4/3 or the multiple antiretroviral drug-resistant virus isolate BE4 was preincubated for 20 min with 50 μg of SEVI and the indicated concentrations of EGCG. Using this mixture, 1G5 cells were subsequently infected for 5 h. At 24 h after infection, luciferase activity was measured as before. (C) Constant amounts (2.5 mM) of EGCG or GC were incubated together with SEVI (5 mg/mL) for the indicated time periods. The degradation of SEVI in each reaction was monitored by Congo red staining (EGCG: black plot; GC: green plot). Subsequently, equal aliquots of each reaction were used to infect 1G5 cells with HIV-1 NL4/3. The respective cultures were assayed as before (EGCG: black bars; GC: green bars).
Fig. 4.
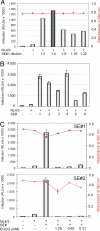
EGCG abrogates SE-enhanced virus infection. (A) TZM-bl cells were infected with 50 ng of p24 antigen of replication-competent HIV-1 NL4/3 in the absence or presence of the indicated dilutions of a randomly selected human SE sample (SE#1). Noninfected cells and infected SE-untreated cells served as controls. Infectivity and cell viability (red plot) was determined essentially as before. (B) Six different SE samples (SE#s 1–6), diluted 1:4 in PBS, were analyzed in parallel as before. Experiments were performed in triplicate. (C) HIV-1 NL4/3 was exposed for 20 min to individual SE samples (SE#s 1 and 2) (1:4 dilution) in the presence or absence of the indicated EGCG concentrations. Subsequently, these mixtures were used for infection of TZM-bl cell cultures. Luciferase activity and cell viability (red plot) were determined at 24 h after infection as before. Error bars represent three independent experiments.
Similar articles
- Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection).
Roan NR, Sowinski S, Münch J, Kirchhoff F, Greene WC. Roan NR, et al. J Biol Chem. 2010 Jan 15;285(3):1861-9. doi: 10.1074/jbc.M109.066167. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897482 Free PMC article. - Epigallocatechin Gallate Inhibits Macaque SEVI-Mediated Enhancement of SIV or SHIV Infection.
Zhou RH, Guo L, Liu JB, Liu H, Hou W, Ma TC, Wang X, Wu JG, Ye L, Ho WZ, Li JL. Zhou RH, et al. J Acquir Immune Defic Syndr. 2017 Jun 1;75(2):232-240. doi: 10.1097/QAI.0000000000001361. J Acquir Immune Defic Syndr. 2017. PMID: 28328549 Free PMC article. - Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI.
Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, Greene WC, Kirchhoff F, Münch J. Kim KA, et al. Retrovirology. 2010 Jun 23;7:55. doi: 10.1186/1742-4690-7-55. Retrovirology. 2010. PMID: 20573198 Free PMC article. - [Semen-derived enhancer of viral infection--a key factor in sexual transmission of HIV].
Duan JM, Qiu JY, Tan SY, Liu SW, Li L. Duan JM, et al. Bing Du Xue Bao. 2012 Jan;28(1):84-8. Bing Du Xue Bao. 2012. PMID: 22416356 Review. Chinese. - Natural Seminal Amyloids as Targets for Development of Synthetic Inhibitors of HIV Transmission.
Sheik DA, Dewhurst S, Yang J. Sheik DA, et al. Acc Chem Res. 2017 Sep 19;50(9):2159-2166. doi: 10.1021/acs.accounts.7b00154. Epub 2017 Aug 15. Acc Chem Res. 2017. PMID: 28809479 Review.
Cited by
- Repurposing Hsp104 to Antagonize Seminal Amyloid and Counter HIV Infection.
Castellano LM, Bart SM, Holmes VM, Weissman D, Shorter J. Castellano LM, et al. Chem Biol. 2015 Aug 20;22(8):1074-86. doi: 10.1016/j.chembiol.2015.07.007. Epub 2015 Aug 6. Chem Biol. 2015. PMID: 26256479 Free PMC article. - Inhibition of the enhancement of infection of human immunodeficiency virus by semen-derived enhancer of virus infection using amyloid-targeting polymeric nanoparticles.
Sheik DA, Brooks L, Frantzen K, Dewhurst S, Yang J. Sheik DA, et al. ACS Nano. 2015 Feb 24;9(2):1829-1836. doi: 10.1021/nn5067254. Epub 2015 Feb 2. ACS Nano. 2015. PMID: 25619867 Free PMC article. - Countering amyloid polymorphism and drug resistance with minimal drug cocktails.
Duennwald ML, Shorter J. Duennwald ML, et al. Prion. 2010 Oct-Dec;4(4):244-51. doi: 10.4161/pri.4.4.13597. Epub 2010 Oct 12. Prion. 2010. PMID: 20935457 Free PMC article. - The amyloidogenic SEVI precursor, PAP248-286, is highly unfolded in solution despite an underlying helical tendency.
Brender JR, Nanga RP, Popovych N, Soong R, Macdonald PM, Ramamoorthy A. Brender JR, et al. Biochim Biophys Acta. 2011 Apr;1808(4):1161-9. doi: 10.1016/j.bbamem.2011.01.010. Epub 2011 Jan 22. Biochim Biophys Acta. 2011. PMID: 21262195 Free PMC article. - Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts.
Chen JC, Johnson BA, Erikson DW, Piltonen TT, Barragan F, Chu S, Kohgadai N, Irwin JC, Greene WC, Giudice LC, Roan NR. Chen JC, et al. Hum Reprod. 2014 Jun;29(6):1255-70. doi: 10.1093/humrep/deu047. Epub 2014 Mar 13. Hum Reprod. 2014. PMID: 24626806 Free PMC article.
References
- UNAIDS. [Accessed November 17, 2008];AIDS epidemic update. 2007 Available at http://data.unaids.org/pub/EPISlides/2007/071118_epi_regional%20factshee....
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. - PubMed
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. - PubMed
- Münch J, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. - PubMed
- Rönnberg L, Vihko P, Sajanti E, Vihko R. Clomiphene citrate administration to normogonadotropic subfertile men: Blood hormone changes and activation of acid phosphatase in seminal fluid. Int J Androl. 1981;4:372–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases