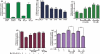Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes - PubMed (original) (raw)
Comparative Study
Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes
Eun-Sook Y Lee et al. J Neurochem. 2009 Jul.
Abstract
Chronic exposure to manganese (Mn) can cause manganism, a neurodegenerative disorder similar to Parkinson's disease. The toxicity of Mn includes impairment of astrocytic glutamate transporters. 17beta-Estradiol (E2) has been shown to be neuroprotective in various neurodegenerative diseases including Parkinson's disease and Alzheimer's disease, and some selective estrogen receptor modulators, including tamoxifen (TX), also possess neuroprotective properties. We have tested our hypothesis that E2 and TX reverse Mn-induced glutamate transporter impairment in astrocytes. The results established that E2 and TX increased glutamate transporter function and reversed Mn-induced glutamate uptake inhibition, primarily via the up-regulation of glutamate/aspartate transporter (GLAST). E2 and TX also increased astrocytic GLAST mRNA levels and attenuated the Mn-induced inhibition of GLAST mRNA expression. In addition, E2 and TX effectively increased the expression of transforming growth factor beta1, a potential modulator of the stimulatory effects of E2/TX on glutamate transporter function. This effect was mediated by the activation of MAPK/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt signaling pathways. These novel findings suggest, for the first time, that E2 and TX enhance astrocytic glutamate transporter expression via increased transforming growth factor beta1 expression. Furthermore, the present study is the first to show that both E2 and TX effectively reverse Mn-induced glutamate transport inhibition by restoring its expression and activity, thus offering a potential therapeutic modality in neurodegenerative disorders characterized by altered glutamate homeostasis.
Figures
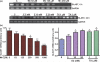
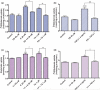
Fig. 1
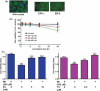
E2/TX protects astrocytes from Mn toxicity in astroglial cultures. (a) Both E2 receptor subtypes, ER-α and ER-β, are expressed in astrocytic cultures. (b) Mn induced astrocytic cytotoxicity occurs in a time- and concentration-dependent manner. Cell viability was assessed by MTT assay as described in the Methods section. (c) E2/TX protects astrocytes from Mn toxicity. Astrocytes were pre-treated for 24 h with E2 (5 and 10 nM) or with TX (0.5 and 1 μM) prior to the addition of Mn (500 μM), followed by the MTT assay. (###p < 0.001 vs. control; **p < 0.01, ***p < 0.001 vs. the Mn treatment; Tukey's test following
anova
). Data are expressed as the mean ± SEM (n = 5–8). Experiments were performed in three independent sets of cultures.
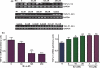
Fig. 2
E2/TX reverses Mn-induced glutamate uptake inhibition in astrocytes. After treatment with Mn or E2/TX, cells were incubated with 100 nM of unlabeled glutamate containing 0.25 μCi of [3H]glutamate (specific activity: 49.0 Ci/mmol) for 10 min. The uptake was terminated by washing the cells with ice-cold PBS buffer as described in the Methods section. (a) Glutamate uptake was THA-sensitive, Na+-dependent and GLAST subtype-sensitive. [3H]glutamate uptake was measured in uptake buffer, Na+-free medium, in the presence of THA (a non-selective inhibitor of glutamate transporters), MSO (an inhibitor of glutamine synthase) or DHK (a selective inhibitor of GLT-1). (MSO, methionine sulfoximine; THA,
dl
-threo-μ-hydroxyaspartic acid; DHK, dihydrokainic acid). (b) Mn (6 h) inhibited glutamate uptake in a concentration-dependent manner. (c) E2/TX (24 h) increased glutamate uptake. (d) E2/TX (18 h pre-treatment) reversed Mn (6 h)-induced glutamate uptake inhibition. (e) E2/TX-induced enhancement of glutamate uptake was ER-dependent. ###p < 0.001 vs. control (d); *p < 0.05, **p < 0.01, ***p < 0.001, ##p < 0.01 vs. control (b,c,e) or Mn treatment (d); Tukey's test following
anova
. Data are expressed as the mean ± SEM (n = 5–8).
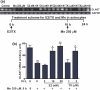
Fig. 3
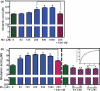
Mn leads to glutamate accumulation in the astrocytic media, whereas E2/TX reverses this effect. Cells were treated for 6 or 24 h with Mn or E2/TX, after which 20 μM glutamate were added to the cell cultures, followed by 30 min of incubation. Glutamate levels in the media were measured by a fluorimetric method. (a) Astrocytes were treated for 6 h with Mn. (b) Astrocytes were treated for 24 h with Mn or E2/TX. For one set of each experiment, cells were pre-treated for 24 h with E2 20 nM prior to Mn (250 μM for 6 or 24 h) treatment. a_p_ < 0.05 vs. control, b_p_ < 0.05 vs. Mn 250 μM, *p < 0.05, **p < 0.01 vs. control; Tukey's test following
anova
. Data are expressed as the mean ± SEM (n = 5–8).
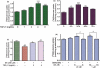
Fig. 4
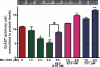
Mn-induced inhibition of GLAST protein expression in whole cell lysates is reversed by E2/TX. (a) Treatment of astrocytes for 6 h with Mn decreased GLAST expression in a concentration-dependent manner. (b) Treatment of astrocytes for 24 h with E2 (20 nM) and TX (1 and 2 μM) increased GLAST protein expression. (c) Pre-treatment of E2 (10 and 20 nM) and TX (1 μM) for 18 h prior to Mn treatment for 6 h attenuated the Mn-induced inhibitory effects of GLAST expression. Western blot data were also quantified. ##p < 0.01, *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following
anova
. Data are expressed as the mean ± SEM (n = 3). (d) Immunocytochemistry study showed that E2 and TX increased astrocytic GLAST expression after 24 h, whereas Mn inhibited GLAST protein expression after 6 h.
Fig. 5
E2/TX enhances GLAST expression on the astroglial plasma membranes and reverses Mn-induced reduction by promoting GLAST trafficking. Astroglial plasma membranes were isolated by biotinylation of surface membrane proteins using a biotinylation kit (Pierce) followed by western blot analysis. All samples were adjusted to contain the same amount of proteins prior to loading on the gel. Mn (500 μM) reduced, while E2/TX increased GLAST expression on astroglial cell surface membranes. E2 reversed the Mn-induced inhibition of GLAST expression (#p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. control; @p < 0.05; Tukey's test following
anova
). Data are expressed as the mean ± SEM (n = 3).
Fig. 6
Mn inhibits, whereas E2/TX increases expression of GLAST mRNA. (a) Incubation of astrocytes for 6 h with Mn decreased the expression of GLAST mRNA, while treatment of astrocytes for 24 h with E2/TX increased GLAST expression by RT-PCR, semi-quantitative analysis of GLAST mRNA. (b) Treatment of astrocytes for 6 h with Mn decreases GLAST expression, whereas treatment of astrocytes for 24 h with E2 and TX increased GLAST mRNA expression by the real-time PCR analysis. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following
anova
. Data are expressed as the mean ± SEM (n = 4).
Fig. 7
Mn and E2/TX exert opposite effects on the expression of astrocytic transforming growth factor β1 (TGF-β1) mRNA. (a) Treatment of astrocytes for 6 h or 24 h with Mn decreased TGF-β1 expression, whereas E2/TX treatment for 24 h increased TGF-β1 expression by RT-PCR analysis. (b) Real time-PCR analysis showed that treatment of astrocytes for 24 h with Mn decreased TGF-β1 mRNA expression, but E2/TX increased TGF-β1 mRNA expression. *p < 0.05, **p < 0.01 ***p < 0.001 vs. control; Tukey's test following
anova
. Data are expressed as the mean ± SEM (n = 4). Experiments were performed in three independent sets of cultures for verification of results.
Fig. 8
E2/TX attenuates the Mn-induced inhibition of GLAST expression in astrocytes. Pre-treatment of astrocytes for 18 h with E2/TX prior to Mn exposure for 6 h protected against the Mn-induced inhibition of GLAST mRNA expression by RT-PCR (a) and real time-PCR analyses (b) (###p < 0.001 vs. control; *p < 0.05, **p < 0.01 vs. the Mn treatment; Tukey's test following
anova
). Data are expressed as the mean ± SEM (n = 4). Experiments were performed in three independent sets of cultures for verification of results.
Fig. 9
TGF-β1 increases glutamate uptake in a concentration- and time-dependent manner in astrocytes. (a) Treatment of astrocytes for 24 h with TGF-β1 (2, 4, 8 ng/mL) increased glutamate uptake. (b) TGF-β1 at 4 ng/mL increased glutamate uptake most effectively after 24 h treatment in astrocytes. (c) Pre-treatment with TGF-β1 (18 h) prior to Mn treatment (6 h) reversed the Mn-induced glutamate uptake inhibition in astrocytes. (d) SB431542 (a TGFR inhibitor, 20 μM) abolished the E2/TX-induced enhancement of glutamate uptake (###p < 0.01 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. control (a,b,d), or the Mn treatment (c), #p < 0.05; Tukey's test following
anova
). Data are expressed as the mean ± SEM (n = 4).
Fig. 10
(a,b) Activation of the MAPK/ERK signaling pathway is required for both E2/TX(a)- and TGF-β1(b)-induced enhancement of glutamate uptake in astrocytes. The addition of PD98059 50 μM, an inhibitor of MAPK/ERK, to the culture media 30 min prior to E2/TX or TGF-β1 treatment for 24 h abolished the E2/TX- or TGF-β1-induced enhancement of glutamate uptake (PD98059 was dissolved in DMSO as a concentration of 6.5 mg/mL and diluted with Opti-MEM experimental media). (c,d) Activation of the PI3K/Akt signaling pathway is required for both E2/TX(c)- and TGF-β1(d)-induced enhancement of glutamate uptake in astrocytes. The addition of LY294002 20 μM, an inhibitor of PI3K/Akt, to the culture media 30 min prior to E2/TX or TGF-β1 treatment for 24 h abolished the E2/TX- or TGF-β1-incuded enhancement of glutamate uptake (LY294002 was dissolved in DMSO at a concentration of 25 mg/mL and diluted with Opti-MEM media). The same concentration of DMSO was used as a control. (#p < 0.05, ##p < 0.01, ###p < 0.001; *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey's test following
anova
). Data are expressed as the mean ± SEM (n = 4).
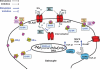
Fig. 11
Proposed mechanism of E2/TX-induced restoration on Mn-induced astroglial glutamate transporter impairment. E2/TX may act via genomic actions to increase the expression of TGF-β1, which in turn increases GLAST expression in astrocytes. This action is ER-dependent. Modulation of GLAST trafficking by Mn or E2/TX may also play a role in the ability of E2/TX to attenuate Mn-induced glutamate transporter inhibition. Activation of MAPK/ERK and PI3K/Akt signaling is required for E2/TX and TGF-β1 in restoring glutamate transporter function (expression). Glu, glutamate; E2, 17β-estradiol; TX, tamoxifen; ER, estrogen receptor; GLAST, glutamate aspartate transporter; TGFR, transforming growth factor receptor.
Similar articles
- Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes.
Lee E, Sidoryk-Wegrzynowicz M, Farina M, Rocha JB, Aschner M. Lee E, et al. Neurotox Res. 2013 Feb;23(2):124-30. doi: 10.1007/s12640-012-9347-2. Epub 2012 Aug 10. Neurotox Res. 2013. PMID: 22878846 Free PMC article. - Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes.
Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Lee ES, et al. Toxicol Sci. 2009 Jul;110(1):156-67. doi: 10.1093/toxsci/kfp081. Epub 2009 Apr 21. Toxicol Sci. 2009. PMID: 19383943 Free PMC article. - Transforming growth factor-α mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes.
Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M. Lee E, et al. Glia. 2012 Jul;60(7):1024-36. doi: 10.1002/glia.22329. Epub 2012 Apr 7. Glia. 2012. PMID: 22488924 Free PMC article. - Role of transcription factor yin yang 1 in manganese-induced reduction of astrocytic glutamate transporters: Putative mechanism for manganese-induced neurotoxicity.
Karki P, Smith K, Johnson J Jr, Aschner M, Lee E. Karki P, et al. Neurochem Int. 2015 Sep;88:53-9. doi: 10.1016/j.neuint.2014.08.002. Epub 2014 Aug 13. Neurochem Int. 2015. PMID: 25128239 Free PMC article. Review. - Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-α in estrogen-induced upregulation of glutamate transporters in astrocytes.
Karki P, Smith K, Johnson J Jr, Lee E. Karki P, et al. Mol Cell Endocrinol. 2014 May 25;389(1-2):58-64. doi: 10.1016/j.mce.2014.01.010. Epub 2014 Jan 18. Mol Cell Endocrinol. 2014. PMID: 24447465 Free PMC article. Review.
Cited by
- Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes.
Lee E, Sidoryk-Wegrzynowicz M, Farina M, Rocha JB, Aschner M. Lee E, et al. Neurotox Res. 2013 Feb;23(2):124-30. doi: 10.1007/s12640-012-9347-2. Epub 2012 Aug 10. Neurotox Res. 2013. PMID: 22878846 Free PMC article. - Effect of glutamate and riluzole on manganese-induced apoptotic cell signaling in neuronally differentiated mouse P19 Cells.
Roth JA, Sridhar S, Singleton ST. Roth JA, et al. Neurochem Int. 2012 Jul;61(1):25-33. doi: 10.1016/j.neuint.2012.04.015. Epub 2012 Apr 21. Neurochem Int. 2012. PMID: 22543103 Free PMC article. - Manganese phosphorylates Yin Yang 1 at serine residues to repress EAAT2 in human H4 astrocytes.
Rizor A, Pajarillo E, Son DS, Aschner M, Lee E. Rizor A, et al. Toxicol Lett. 2022 Feb 1;355:41-46. doi: 10.1016/j.toxlet.2021.11.007. Epub 2021 Nov 17. Toxicol Lett. 2022. PMID: 34800614 Free PMC article. - Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway.
Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Sidoryk-Wegrzynowicz M, et al. Glia. 2011 Nov;59(11):1732-43. doi: 10.1002/glia.21219. Epub 2011 Aug 2. Glia. 2011. PMID: 21812036 Free PMC article. - Tamoxifen induces protection against manganese toxicity by REST upregulation via the ER-α/Wnt/β-catenin pathway in neuronal cells.
Digman A, Pajarillo E, Kim S, Ajayi I, Son DS, Aschner M, Lee E. Digman A, et al. J Biol Chem. 2025 Apr 23;301(6):108529. doi: 10.1016/j.jbc.2025.108529. Online ahead of print. J Biol Chem. 2025. PMID: 40280417 Free PMC article.
References
- Abe K, Saito H. Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. J. Neurochem. 2001;76:217–223. - PubMed
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 1992;58:730–735. - PubMed
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS). Neurotoxicology. 1999;20:173–180. - PubMed
- Azcoitia I, Garcia-Ovejero D, Chowen JA, Garcia-Segura LM. Astroglia play a key role in the neuroprotective actions of estrogen. Prog. Brain Res. 2001;132:469–478. - PubMed
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology. 1984;5:13–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous