A familial mutation renders atrial natriuretic Peptide resistant to proteolytic degradation - PubMed (original) (raw)
A familial mutation renders atrial natriuretic Peptide resistant to proteolytic degradation
Deborah M Dickey et al. J Biol Chem. 2009.
Abstract
A heterozygous frameshift mutation causing a 12-amino acid extension to the C terminus of atrial natriuretic peptide (ANP) was recently genetically linked to patients with familial atrial fibrillation (Hodgson-Zingman, D. M., Karst, M. L., Zingman, L. V., Heublein, D. M., Darbar, D., Herron, K. J., Ballew, J. D., de Andrade, M., Burnett, J. C., Jr., and Olson, T. M. (2008) N. Engl. J. Med. 359, 158-165). The frameshift product (fsANP), but not wild-type ANP (wtANP), was elevated in the serum of affected patients, but the molecular basis for the elevated peptide concentrations was not determined. Here, we measured the ability of fsANP to interact with natriuretic peptide receptors and to be proteolytically degraded. fsANP and wtANP bound and activated human NPR-A and NPR-C similarly, whereas fsANP had a slightly increased efficacy for human NPR-B. Proteolytic susceptibility was addressed with novel bioassays that measure the time required for kidney membranes or purified neutral endopeptidase to abolish ANP-dependent activation of NPR-A. The half-life of fsANP was markedly greater than that of wtANP in both assays. Additional membrane proteolysis studies indicated that wtANP and fsANP are preferentially degraded by neutral endopeptidase and serine peptidases, respectively. These data indicate that the familial ANP mutation associated with atrial fibrillation has only minor effects on natriuretic peptide receptor interactions but markedly modifies peptide proteolysis.
Figures
FIGURE 1.
Cartoon schematic of the primary amino acid structure of human atrial natriuretic peptide (wtANP) and the primary amino acid structure of the ANP frameshift mutation (fsANP). The 12-amino acid extension of fsANP is shaded in light gray. The dark gray shading indicates residues conserved in all natriuretic peptides. The black bars indicate disulfide bonds.
FIGURE 2.
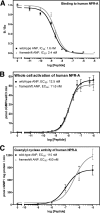
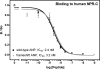
fsANP binds and activates human NPR-A similarly to wtANP. A, competition 125I-ANP binding to 293 cells stably expressing human NPR-A. Binding of the radioligand was displaced with varying concentrations of either nonradiolabeled fsANP or nonradiolabeled wild-type ANP. The results are the means of three separate experiments conducted in triplicate ± S.E. where n = 9. B, 3-min stimulations of 293neo-hNPR-A cells stably overexpressing human NPR-A. The results are the means of five experiments ± S.E. where n = 15. C, 3-min guanylyl cyclase activity measurements in membrane preparations from 293neo-hNPR-A cells stably overexpressing human NPR-A. The results are the means of three separate experiments performed in triplicate ± S.E. where n = 9. p values were not significant.
FIGURE 3.
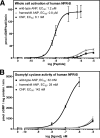
fsANP has reduced potency for rat NPR-A. A, 3-min stimulations of 293-GCA cells stably overexpressing rat NPR-A. The results are the means of three experiments ± S.E. where n = 9. Each individual experiment contained three data points, where the p value = 0.02. B, competition 125I-ANP binding experiments using unlabeled rat ANP, wtANP, or fsANP to compete for binding to cells stably expressing rat NPR-A. The results are the means of three separate experiments conducted in triplicate + S.E. where n = 9, and the p value for human ANP versus fsANP = 0.001.
FIGURE 4.
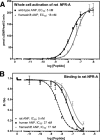
fsANP is a more efficacious activator of human NPR-B than wtANP. A, 293 cells stably expressing human NPR-B were incubated with wtANP, fsANP, or CNP for 3 min, and then intracellular cGMP concentrations were determined by radioimmunoassay. The results are the means of five separate experiments ± S.E., where n = 15. The p value for human ANP versus fsANP equals 0.01. B, natriuretic peptide-dependent guanylyl cyclase assays were conducted on membrane preparations from 293 cells stably expressing human NPR-B. The results are the means of three separate experiments conducted in triplicate ± S.E. where n = 9. The p value was > 0.05.
FIGURE 5.
fsANP and wtANP bind human NPR-C similarly. Competition binding of 125I-ANP to 293 cells stably expressing human NPR-C. The results are the means of three separate experiments conducted in triplicate ± S.E. where n = 9. The p value was > 0.05.
FIGURE 6.
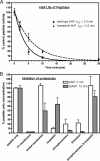
fsANP is resistant to protease-dependent degradation and has a different protease susceptibility profile than wtANP. A shows the ability of human kidney membranes to degrade wt or fsANP. 1 μ
m
concentrations of each peptide were incubated with 40 μg of human kidney membranes for the designated periods of time at 37 °C in a total volume of 0.1 ml. Proteolysis was terminated by addition of 0.1 ml of 0.5
m
perchloric acid, and bioactivity was evaluated by measuring the ability of the neutralized extracts to elevate cGMP concentration in HEK 293 cells stably expressing human NPR-A. The error bars represent the S.E. where n = 4. B shows the effect of blocking human kidney membrane-dependent degradation of wt or fsANP peptide by boiling the membranes for 30 min prior to exposure to peptide or by including various protease inhibitors in the proteolysis assay. Each point represents the mean ± S.E. where n = 12.
FIGURE 7.
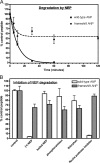
fsANP is resistant to NEP-dependent degradation. A, inactivation/degradation of wt or fsANP by purified human recombinant NEP. The error bars represent the S.E. where n = 4. B, inhibition of NEP-dependent inactivation of wt or fsANP by boiling NEP for 30 min prior to peptide exposure or by including NEP-specific protease inhibitors (phosphoramidon or thiorphan) in the proteolysis assay. Each point represents the mean ± S.E. where n = 4.
Similar articles
- Receptor-specific ligands distinguish natriuretic peptide receptors-A and -C in primate tissues.
Sehl P, Tom JY, Oare D, Lowe DG. Sehl P, et al. Mol Cell Biochem. 1998 Jan;178(1-2):317-24. doi: 10.1023/a:1006823732336. Mol Cell Biochem. 1998. PMID: 9546616 - Differential regulation of natriuretic peptide receptors on ciliary body epithelial cells.
Crook RB, Chang AT. Crook RB, et al. Biochem J. 1997 May 15;324 ( Pt 1)(Pt 1):49-55. doi: 10.1042/bj3240049. Biochem J. 1997. PMID: 9164840 Free PMC article. - A constitutively "phosphorylated" guanylyl cyclase-linked atrial natriuretic peptide receptor mutant is resistant to desensitization.
Potter LR, Hunter T. Potter LR, et al. Mol Biol Cell. 1999 Jun;10(6):1811-20. doi: 10.1091/mbc.10.6.1811. Mol Biol Cell. 1999. PMID: 10359598 Free PMC article. - [Molecular biology and pharmacology of natriuretic peptide system].
Itoh H, Suga S, Ogawa Y, Tanaka I, Nakao K. Itoh H, et al. Nihon Rinsho. 1993 Jun;51(6):1548-56. Nihon Rinsho. 1993. PMID: 8100590 Review. Japanese. - Natriuretic peptide receptor-C signaling and regulation.
Anand-Srivastava MB. Anand-Srivastava MB. Peptides. 2005 Jun;26(6):1044-59. doi: 10.1016/j.peptides.2004.09.023. Epub 2005 Apr 8. Peptides. 2005. PMID: 15911072 Review.
Cited by
- Discovery of small molecule guanylyl cyclase A receptor positive allosteric modulators.
Sangaralingham SJ, Whig K, Peddibhotla S, Kirby RJ, Sessions HE, Maloney PR, Hershberger PM, Mose-Yates H, Hood BL, Vasile S, Pan S, Zheng Y, Malany S, Burnett JC Jr. Sangaralingham SJ, et al. Proc Natl Acad Sci U S A. 2021 Dec 28;118(52):e2109386118. doi: 10.1073/pnas.2109386118. Proc Natl Acad Sci U S A. 2021. PMID: 34930837 Free PMC article. - Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.
Potter LR. Potter LR. Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review. - Novel enhancers of guanylyl cyclase-A activity acting via allosteric modulation.
Andresen H, Pérez-Ternero C, Robinson J, Dickey DM, Hobbs AJ, Potter LR, Levy FO, Cataliotti A, Moltzau LR. Andresen H, et al. Br J Pharmacol. 2023 Dec;180(24):3254-3270. doi: 10.1111/bph.16203. Epub 2023 Aug 29. Br J Pharmacol. 2023. PMID: 37522273 Free PMC article. - Decreased plasma musclin levels are associated with potential atrial fibrillation in non-diabetic patients.
Zhong Y, Zhang J, Tang K, Kou W, Xu S, Yang H, Liu L, Luan P, Mohammed AQ, Abdu FA, Zhao D, Li H, Peng W, Xu Y. Zhong Y, et al. Ann Transl Med. 2021 Feb;9(3):203. doi: 10.21037/atm-20-3259. Ann Transl Med. 2021. PMID: 33708830 Free PMC article. - A State of Natriuretic Peptide Deficiency.
Nyberg M, Terzic D, Ludvigsen TP, Mark PD, Michaelsen NB, Abildstrøm SZ, Engelmann M, Richards AM, Goetze JP. Nyberg M, et al. Endocr Rev. 2023 May 8;44(3):379-392. doi: 10.1210/endrev/bnac029. Endocr Rev. 2023. PMID: 36346821 Free PMC article.
References
- Potter L. R., Abbey-Hosch S., Dickey D. M. (2006) Endocr. Rev. 27, 47–72 - PubMed
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. (1989) Nature 338, 78–83 - PubMed
- Suga S., Nakao K., Hosoda K., Mukoyama M., Ogawa Y., Shirakami G., Arai H., Saito Y., Kambayashi Y., Inouye K., Imura H. (1992) Endocrinology 130, 229–239 - PubMed
- Lopez M. J., Garbers D. L., Kuhn M. (1997) J. Biol. Chem. 272, 23064–23068 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous