Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy - PubMed (original) (raw)
Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy
Xiaoming Zhou et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Perinuclear aggresome formation is a key mechanism to dispose of misfolded proteins that exceed the degradative capacity of ubiquitin-proteasome and autophagy-lysosome systems. Functional blockade of either degradative system leads to an enhanced aggresome formation. The tuberous sclerosis complex-Ras homologue enriched in brain-mammalian target of rapamycin (TSC-Rheb-mTOR) pathway is known to play a central role in modulating protein synthesis and autophagy. However, in spite of the constitutive activation of mTOR and the abrogated autophagy activity in TSC1- or TSC2-deficient cells, the TSC mutant cells are defective in aggresome formation and undergo apoptosis upon misfolded protein accumulation both in vitro and in vivo. High Rheb activity in TSC mutant cells inhibits aggresome formation and sensitizes cell death in response to misfolded proteins. Surprisingly, this previously unrecognized function of Rheb is independent of TOR complex 1. Active Rheb disrupts the interaction between dynein and misfolded protein cargos, and therefore blocks aggresome formation by inhibiting dynein-dependent transportation of misfolded proteins. This study reveals a function of Rheb in controlling misfolded protein metabolism by modulating aggresome formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
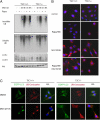
TSC1−/− MEF is defective in aggresome formation. (A) TSC1−/− cells have less insoluble ubiquitinated protein, even in the presence of rapamycin (Rapa). TSC1−/− MEF was a spontaneously immortalized MEF cell line derived from TSC1−/− embryos. TSC1+/+ and TSC1−/− MEFs with or without 20 nM rapamycin pretreatment for 36 h were treated with DMSO or 5 μM MG132 for 4 or 9 h before harvest. RIPA-insoluble cell lysates were normalized and blotted for ubiquitin (UB). RIPA-soluble lysates were blotted for UB, LC3, and Akt (for loading control). (B) Rapamycin does not restore aggresome formation in TSC1−/− cells. TSC1+/+ and TSC1−/− MEFs with or without 20 nM rapamycin pretreatment for 36 h were treated with DMSO or 5 μM MG132 for 9 h before fixation. Cells were immunostained for ubiquitin–protein conjugates (red), and nuclei were counterstained with DAPI (blue). (C) TSC1−/− cells are defective in autophagy and aggresome formation. TSC1+/+, TSC1−/− MEFs expressing EGFP-LC3 (green) treated with 5 μM MG132 or DMSO for 10 h were immunostained for ubiquitin–protein conjugates. Nuclei were stained with DAPI. The data show that LC3 signals encroach aggresome in TSC1+/+ but not in TSC1−/− cells. O/L denotes overlay.
Fig. 2.
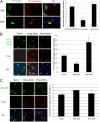
Rheb regulates ubiquitinated aggresome formation. (A) Rheb regulates endogenous aggresome formation. YFP-Rheb-Q64L (constitutively active) or YFP-Rheb-D60K (dominant-negative) constructs were transfected into A549 cells, followed by 5 μM MG132 treatment for 12 h. (Left) Cells were immunostained for ubiquitin (UB)–protein conjugates, and nuclei were stained with DAPI. (Right) Percentage of aggresome-harboring cells was counted among the transfectants and nontransfectants. Values represent means ± SD of 3 independent experiments. **, P < 0.01 (Student's t test). (B) Rheb regulates aggregation of CFTR-ΔF508. At 18 h after cotransfection of EGFP-CFTRΔF508 with myc-Rheb-Q64L, myc-Rheb-D60K, or control vector, COS-7 cells were immunostained for MYC and nuclei (DAPI). (Left) Representative images are shown from 3 independent experiments. (Right) Percentage of aggresome-harboring cells was scored among the cotransfectants. Values represent means ± SD of 3 independent experiments. *, P < 0.05. (C) Rheb does not regulate nonubiquitinated aggresome formation. (Left) At 18 h after cotransfection of GFP-250 plasmids with myc-Rheb-Q64L, myc-Rheb-D60K, or vector constructs, COS-7 cells were immunostained for MYC and nuclei (DAPI). (Right) Percentage of aggresome-harboring cells was scored among the cotransfectants as shown. Values represent means ± SD of 3 independent experiments. O/L denotes overlay.
Fig. 3.
TSC1 deletion and Rheb activation sensitize misfolded protein-induced apoptosis. (A) TSC1−/− cells are sensitive to MG132. TSC1+/+ and TSC1−/− MEFs with or without 20 nM rapamycin (Rapa) pretreatment for 1 h were challenged with 5 μM MG132 or DMSO for 12 h. Phase-contrast images were taken. (B) MG132 induces apoptosis in TSC1−/− cells. TSC1+/+ and TSC1−/− MEFs with or without 20 nM rapamycin pretreatment for 1 h were challenged with 5 μM MG132 or DMSO for 4 or 9 h before harvest. Cell lysates were blotted for cleaved caspase-3, cleaved PARP, and Akt (for loading control). (C) Rheb-Q64L sensitizes cell death to MG132. (Left) MCF-7 stable cell clones expressing YFP or YFP-Rheb-Q64L treated with 5 μM MG132 for 24 h were immunostained with cleaved caspase-3 antibody, and nuclei were stained with DAPI. (Right) Percentage of cleaved caspase-3 positively stained cells for different clones is shown in diagram.
Fig. 4.
TSC1 knockout causes defective aggresome formation and sensitizes misfolded protein-induced apoptosis. (A) TSC1 knockout hepatocytes are compromised in aggresome formation. Shown are representative images of ubiquitin–protein conjugate immunostaining and nuclei staining (DAPI) of liver frozen sections from TSC1flox/flox (F/F) and TSC1flox/flox, albumin-Cre (K/O) littermates with or without (control) PS341 treatment. (B) PS341 induces apoptosis in TSC1−/− liver. PS341 preferentially induces apoptosis in TSC1−/− hepatocytes. Liver homogenates from TSC1flox/flox (F/F) and TSC1flox/flox, albumin-Cre (K/O) littermates with or without (control) PS341 treatment were immunoblotted with TSC1, TSC2, cleaved caspase-3, PARP, and α-tubulin.
Fig. 5.
High Rheb activity inhibits the interaction between the dynein motor and ubiquitinated protein cargos. (A) The association between dynein and ubiquitinated proteins is disrupted by TSC1 deletion. TSC1+/+, TSC1−/− MEFs with or without 20 nM rapamycin (Rapa) pretreatment for 1 h were treated with 5 μM MG132 or DMSO for 4 h before harvest. Lysates were normalized and immunoprecipitated (IP) with dynein antibody. Immunoprecipitates were immunoblotted with antibodies for ubiquitin (UB) and dynein. Whole-cell lysates were blotted for P-S6K (T389) and p70 S6K. (B) Rheb knockdown increases cell viability in response to MG132. TSC1 MEFs lentivirally introduced with Rheb shRNA or scramble shRNA were challenged with 5 μM MG132 at 9 h before harvest. RIPA-insoluble cell lysates were normalized and blotted for UB. Soluble lysates were blotted for UB, Rheb, and cleaved caspase-3. (C) A proposed model for TSC1/2–Rheb–mTOR in the regulation of misfolded protein metabolism.
Similar articles
- Non-canonical functions of the tuberous sclerosis complex-Rheb signalling axis.
Neuman NA, Henske EP. Neuman NA, et al. EMBO Mol Med. 2011 Apr;3(4):189-200. doi: 10.1002/emmm.201100131. Epub 2011 Mar 16. EMBO Mol Med. 2011. PMID: 21412983 Free PMC article. Review. - The TSC1 and TSC2 tumor suppressors are required for proper ER stress response and protect cells from ER stress-induced apoptosis.
Kang YJ, Lu MK, Guan KL. Kang YJ, et al. Cell Death Differ. 2011 Jan;18(1):133-44. doi: 10.1038/cdd.2010.82. Epub 2010 Jul 9. Cell Death Differ. 2011. PMID: 20616807 Free PMC article. - Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner.
Banerjee S, Crouse NR, Emnett RJ, Gianino SM, Gutmann DH. Banerjee S, et al. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15996-6001. doi: 10.1073/pnas.1019012108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896734 Free PMC article. - TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity.
Yang Q, Inoki K, Kim E, Guan KL. Yang Q, et al. Proc Natl Acad Sci U S A. 2006 May 2;103(18):6811-6. doi: 10.1073/pnas.0602282103. Epub 2006 Apr 20. Proc Natl Acad Sci U S A. 2006. PMID: 16627617 Free PMC article. - The TSC1-TSC2 complex: a molecular switchboard controlling cell growth.
Huang J, Manning BD. Huang J, et al. Biochem J. 2008 Jun 1;412(2):179-90. doi: 10.1042/BJ20080281. Biochem J. 2008. PMID: 18466115 Free PMC article. Review.
Cited by
- Recruitment of the oncoprotein v-ErbA to aggresomes.
Bondzi C, Brunner AM, Munyikwa MR, Connor CD, Simmons AN, Stephens SL, Belt PA, Roggero VR, Mavinakere MS, Hinton SD, Allison LA. Bondzi C, et al. Mol Cell Endocrinol. 2011 Jan 30;332(1-2):196-212. doi: 10.1016/j.mce.2010.10.012. Epub 2010 Nov 12. Mol Cell Endocrinol. 2011. PMID: 21075170 Free PMC article. - Recent progress in the study of the Rheb family GTPases.
Heard JJ, Fong V, Bathaie SZ, Tamanoi F. Heard JJ, et al. Cell Signal. 2014 Sep;26(9):1950-7. doi: 10.1016/j.cellsig.2014.05.011. Epub 2014 May 24. Cell Signal. 2014. PMID: 24863881 Free PMC article. Review. - Metformin induces degradation of mTOR protein in breast cancer cells.
Alalem M, Ray A, Ray BK. Alalem M, et al. Cancer Med. 2016 Nov;5(11):3194-3204. doi: 10.1002/cam4.896. Epub 2016 Oct 17. Cancer Med. 2016. PMID: 27748082 Free PMC article. - Lymphangioleiomyomatosis - a wolf in sheep's clothing.
Henske EP, McCormack FX. Henske EP, et al. J Clin Invest. 2012 Nov;122(11):3807-16. doi: 10.1172/JCI58709. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114603 Free PMC article. Review. - Mammalian target of rapamycin signaling and autophagy: roles in lymphangioleiomyomatosis therapy.
Yu J, Parkhitko AA, Henske EP. Yu J, et al. Proc Am Thorac Soc. 2010 Feb;7(1):48-53. doi: 10.1513/pats.200909-104JS. Proc Am Thorac Soc. 2010. PMID: 20160148 Free PMC article. Review.
References
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. - PubMed
- Kawaguchi Y, et al. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. - PubMed
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous