Anaplastic lymphoma kinase: signalling in development and disease - PubMed (original) (raw)
Review
Anaplastic lymphoma kinase: signalling in development and disease
Ruth H Palmer et al. Biochem J. 2009.
Abstract
RTKs (receptor tyrosine kinases) play important roles in cellular proliferation and differentiation. In addition, RTKs reveal oncogenic potential when their kinase activities are constitutively enhanced by point mutation, amplification or rearrangement of the corresponding genes. The ALK (anaplastic lymphoma kinase) RTK was originally identified as a member of the insulin receptor subfamily of RTKs that acquires transforming capability when truncated and fused to NPM (nucleophosmin) in the t(2;5) chromosomal rearrangement associated with ALCL (anaplastic large cell lymphoma). To date, many chromosomal rearrangements leading to enhanced ALK activity have been described and are implicated in a number of cancer types. Recent reports of the EML4 (echinoderm microtubule-associated protein like 4)-ALK oncoprotein in NSCLC (non-small cell lung cancer), together with the identification of activating point mutations in neuroblastoma, have highlighted ALK as a significant player and target for drug development in cancer. In the present review we address the role of ALK in development and disease and discuss implications for the future.
Figures
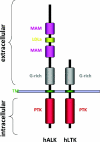
Figure 1. Domain structure of human ALK and human LTK
The N-terminal region of human ALK (hALK) comprises two MAM domains (amino acids 264–427 and 480–626), one LDLa domain (amino acids 453–471) and a glycine rich (G-rich) region (amino acids 816–940). A transmembrane (TM)-spanning segment, connects the extracellular region with the protein tyrosine kinase (PTK), domain (amino acids 1116–1383)-containing intracellular region. The closest family member, LTK [hLTK (human LTK)], is depicted with the corresponding regions denoted. The signal peptide (amino acids 1–16), the glycine rich, G-rich, domain (amino acids 63–334) and the kinase domain (amino acids 510–777) located in the intracellular C-terminal region of the protein.
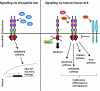
Figure 2. Signalling via ALK
Signalling via dALK occurs via binding of the ligand Jeb, downstream activation of ERK and transcription of downstream target genes. Signalling via mammalian ALK is thought to occur via ligand-mediated dimerization in response to the MK and PTN ligands. ALK mediates signalling via the JAK/STAT, RAS/MAPK, PI3K and PLCγ pathways. Activation of ALK via RPTPβ/ζ, independently of direct ALK–ligand interactions has also been proposed. Lastly, ALK is proposed to function as a dependency receptor which is cleaved by caspase 3 (Casp. 3) in the absence of ligand, thereby promoting apoptosis.
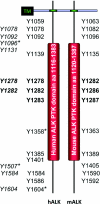
Figure 3. Tyrosine residues phosphorylated in the intracellular regions of human and mouse ALK
The intracellular regions of human and mouse ALK (hALK and mALK respectively) contain the PTK domain. Potential autophosphorylation sites are shown within human and mouse ALK [11]. Note that there is no equivalent tyrosine residue in mouse ALK for human Tyr1604. Tyrosine residues within the activation loop are shown in bold. In italics is a profiling of putative phosphorylated tyrosine residues in NPM–ALK from ALCL cancer cells [166]. Four tyrosine sites, marked with*, have been tested by mutagenesis in NPM–ALK for interaction with signalling proteins such as PLC-γ (Tyr1604 in hALK) [11], Shc (Tyr1507 in hALK) [115], Src (Tyr1358 in hALK) [157], IRS-1 (Tyr1096 in hALK) [115] and SNT (Tyr1096 and Tyr1507 in hALK) [37]. Localization of tyrosine residues within the intracellular region is not to scale. TM, transmembrane domain.
Similar articles
- Anaplastic lymphoma kinase as a therapeutic target in anaplastic large cell lymphoma, non-small cell lung cancer and neuroblastoma.
Cheng M, Ott GR. Cheng M, et al. Anticancer Agents Med Chem. 2010 Mar;10(3):236-49. doi: 10.2174/1871520611009030236. Anticancer Agents Med Chem. 2010. PMID: 20406193 Review. - Nucleophosmin-anaplastic lymphoma kinase: the ultimate oncogene and therapeutic target.
Werner MT, Zhao C, Zhang Q, Wasik MA. Werner MT, et al. Blood. 2017 Feb 16;129(7):823-831. doi: 10.1182/blood-2016-05-717793. Epub 2016 Nov 22. Blood. 2017. PMID: 27879258 Review. - Involvement of Grb2 adaptor protein in nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)-mediated signaling and anaplastic large cell lymphoma growth.
Riera L, Lasorsa E, Ambrogio C, Surrenti N, Voena C, Chiarle R. Riera L, et al. J Biol Chem. 2010 Aug 20;285(34):26441-50. doi: 10.1074/jbc.M110.116327. Epub 2010 Jun 16. J Biol Chem. 2010. PMID: 20554525 Free PMC article. - The emerging pathogenic and therapeutic importance of the anaplastic lymphoma kinase gene.
Kelleher FC, McDermott R. Kelleher FC, et al. Eur J Cancer. 2010 Sep;46(13):2357-68. doi: 10.1016/j.ejca.2010.04.006. Epub 2010 May 5. Eur J Cancer. 2010. PMID: 20451371 Review. - Molecular characterization of the t(2;5) (p23; q35) translocation in anaplastic large cell lymphoma (Ki-1) and Hodgkin's disease.
Yee HT, Ponzoni M, Merson A, Goldstein M, Scarpa A, Chilosi M, Menestrina F, Pittaluga S, de Wolf-Peeters C, Shiota M, Mori S, Frizzera G, Inghirami G. Yee HT, et al. Blood. 1996 Feb 1;87(3):1081-8. Blood. 1996. PMID: 8562933
Cited by
- New insights into the genetics of neuroblastoma.
Sridhar S, Al-Moallem B, Kamal H, Terrile M, Stallings RL. Sridhar S, et al. Mol Diagn Ther. 2013 Apr;17(2):63-9. doi: 10.1007/s40291-013-0019-6. Mol Diagn Ther. 2013. PMID: 23329364 Review. - Unraveling the Potential of ALK-Targeted Therapies in Non-Small Cell Lung Cancer: Comprehensive Insights and Future Directions.
Parvaresh H, Roozitalab G, Golandam F, Behzadi P, Jabbarzadeh Kaboli P. Parvaresh H, et al. Biomedicines. 2024 Jan 27;12(2):297. doi: 10.3390/biomedicines12020297. Biomedicines. 2024. PMID: 38397899 Free PMC article. Review. - A Need for More Molecular Profiling in Brain Metastases.
Shen E, Van Swearingen AED, Price MJ, Bulsara K, Verhaak RGW, Baëta C, Painter BD, Reitman ZJ, Salama AKS, Clarke JM, Anders CK, Fecci PE, Goodwin CR, Walsh KM. Shen E, et al. Front Oncol. 2022 Jan 25;11:785064. doi: 10.3389/fonc.2021.785064. eCollection 2021. Front Oncol. 2022. PMID: 35145903 Free PMC article. Review. - Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study.
Reynolds C, Masters ET, Black-Shinn J, Boyd M, Mardekian J, Espirito JL, Chioda M. Reynolds C, et al. J Clin Med. 2018 May 29;7(6):129. doi: 10.3390/jcm7060129. J Clin Med. 2018. PMID: 29844259 Free PMC article. - An evolutionary conserved role for anaplastic lymphoma kinase in behavioral responses to ethanol.
Lasek AW, Lim J, Kliethermes CL, Berger KH, Joslyn G, Brush G, Xue L, Robertson M, Moore MS, Vranizan K, Morris SW, Schuckit MA, White RL, Heberlein U. Lasek AW, et al. PLoS One. 2011;6(7):e22636. doi: 10.1371/journal.pone.0022636. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799923 Free PMC article.
References
- Morris S. W., Kirstein M. N., Valentine M. B., Dittmer K. G., Shapiro D. N., Saltman D. L., Look A. T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. - PubMed
- Shiota M., Fujimoto J., Semba T., Satoh H., Yamamoto T., Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9:1567–1574. - PubMed
- Iwahara T., Fujimoto J., Wen D., Cupples R., Bucay N., Arakawa T., Mori S., Ratzkin B., Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. - PubMed
- Morris S. W., Naeve C., Mathew P., James P. L., Kirstein M. N., Cui X., Witte D. P. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous