Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia - PubMed (original) (raw)
Review
Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia
Shinae Kizaka-Kondoh et al. Cancer Sci. 2009 Aug.
Abstract
A tumor-specific microenvironment is characterized by hypoxia, in which oxygen tension is considerably lower than in normal tissues. The hypoxic status of various solid tumors has been attributed as an indicator of adverse prognosis due to tumor progression toward a more malignant phenotype with increased metastatic potential and resistance to treatment. Various exogenous and endogenous markers for hypoxia are currently available and studied in relation to each other, tumor architecture, and tumor microenvironment. Over the last few decades, various methods have been suggested to assess the level of oxygenation in solid tumors. Among them, nitroimidazole compounds have provided promising information on tumor hypoxia. To quantify the extent of hypoxia requires that nitroimidazole binding be primarily dependent on oxygen concentration as well as nitroreductase levels in the tumor cells. Furthermore, recent progress in molecular biology has highlighted a transcription factor, hypoxia-inducible factor (HIF)-1, whose activity is induced by hypoxia. HIF-1 plays a central role in malignant progression by inducing the expression of various genes, whose functions are strongly associated with malignant alteration of the entire tumor. The cellular changes induced by HIF-1 are extremely important therapeutic targets of cancer therapy, particularly in the therapy against refractory cancers. In this review, we will discuss the significance of pimonidazole and HIF-1 as exogenous and endogenous hypoxia markers, respectively, as well as their evaluation and imaging of tumor hypoxia.
Figures
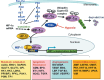
Figure 1
Regulation of hypoxia‐inducible factor (HIF)‐1. In the presence of oxygen (normoxia), prolyl hydroxylase (PHD) hydroxylates proline residues on HIF‐1α, allowing HIF‐1α to interact with a ubiquitin–protein ligase complex (VHL, CLU2, and Elongin‐B and Elongin‐C) through VHL. Ubiquitination of HIF‐1α makes it a target for degradation by the 26S proteasome. Growth signals through receptor tyrosine kinases (Rec‐Tyr) and Ras activate the PI3K–Akt pathway and the Ras–Raf–MAP kinase pathway, respectively, increasing the translation of HIF‐1α. When oxygen supply is not enough to activate PHD or when HIF‐1α expression exceeds the capacity of ubiquitin–proteasome degradation, HIF‐1α binds to ubiquitously expressing HIF‐1β to form a heterodimer. The heterodimer then translocates to the nucleus and binds to hypoxia‐responsive elements in the promoter and enhancer region of target genes, inducing the expression of various HIF1‐responsive genes. HRE, hypoxia‐responsive element; Ub, ubiquitin.
Figure 2
Tumor microenvironment. Tumor hypoxia arises in regions with impaired oxygen delivery. The regions proximal to blood vessels are Ki‐67‐, FDG‐, and glucose transporter (Glut)‐1‐positive but radiosensitive, whereas the diffusion‐limited regions are Ki‐67‐, FDG‐, and Glut‐1‐negative and radioresistant. In hypoxic regions, HIF‐1‐active regions (red/pink) are located closer to the blood vessels than pimonidazole (Pimo)‐positive regions (light green). Pimo‐positive regions are located next to necrotic regions (dark blue) and barely express HIF‐1α; they possess little HIF‐1 activity. CA9, carbonic anhydrase 9; FMISO, fluoromisonidazole.
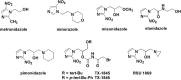
Figure 3
Representative hypoxic cell radiosensitizers and bioreductive prodrugs containing a nitroheterocyclic group.
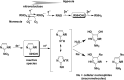
Figure 4
Oxygen‐dependent bioreductive metabolism of nitroimidazoles in cells and proposed mechanism for selective covalent binding reaction of hydroxylamine intermediates with cellular nucleophiles under hypoxic conditions.
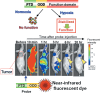
Figure 5
Imaging of hypoxia‐inducible factor (HIF)‐1 active cells. (a) Protein transduction domain (PTD)–oxygen‐dependent degradation (ODD) fusion protein consisting of three domains: PTD, ODD, and a functional domain. PTD enables the fusion protein to diffuse and enter the cell. ODD is derived from ODD548–603 of the HIF‐1α protein and endows the fusion protein with the same oxygen‐dependent degradation regulation as the HIF‐1α protein. Thus, PTD–ODD is degraded quickly in normoxia (aerobic conditions) but is stabilized and functional in hypoxia. (b) The PTD–ODD–enhanced GFP (EGFP) fusion protein (probe) was labeled with a near‐infrared fluorescent dye and injected into a tumor‐bearing mouse. Fluorescence was detected in the whole body shortly after i.v. injection of the labeled probe. By 6 h after probe injection, the fluorescence was predominantly detected in the tumor, suggesting that the PTD–ODD probe could be a potential probe for imaging HIF‐1 activity.
Similar articles
- Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers.
Bussink J, Kaanders JH, van der Kogel AJ. Bussink J, et al. Radiother Oncol. 2003 Apr;67(1):3-15. doi: 10.1016/s0167-8140(03)00011-2. Radiother Oncol. 2003. PMID: 12758235 Review. - Comparison between pimonidazole binding, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix.
Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, MacPhail SH, Olive PL. Jankovic B, et al. Cytometry B Clin Cytom. 2006 Mar;70(2):45-55. doi: 10.1002/cyto.b.20086. Cytometry B Clin Cytom. 2006. PMID: 16456867 - The HIF-1-active microenvironment: an environmental target for cancer therapy.
Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. Kizaka-Kondoh S, et al. Adv Drug Deliv Rev. 2009 Jul 2;61(7-8):623-32. doi: 10.1016/j.addr.2009.01.006. Epub 2009 May 3. Adv Drug Deliv Rev. 2009. PMID: 19409433 Review. - Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge.
Koyasu S, Kobayashi M, Goto Y, Hiraoka M, Harada H. Koyasu S, et al. Cancer Sci. 2018 Mar;109(3):560-571. doi: 10.1111/cas.13483. Epub 2018 Jan 27. Cancer Sci. 2018. PMID: 29285833 Free PMC article. Review. - Reduced expression of hypoxia-inducible factor-1alpha in perinecrotic regions of solid tumors.
Sobhanifar S, Aquino-Parsons C, Stanbridge EJ, Olive P. Sobhanifar S, et al. Cancer Res. 2005 Aug 15;65(16):7259-66. doi: 10.1158/0008-5472.CAN-04-4480. Cancer Res. 2005. PMID: 16103077
Cited by
- Factors Predicting Effectiveness of Neoadjuvant Therapy for Esophageal Squamous Cell Carcinoma.
Ohkura Y, Ueno M, Iizuka T, Haruta S, Tanaka T, Udagawa H. Ohkura Y, et al. Medicine (Baltimore). 2016 Apr;95(15):e3365. doi: 10.1097/MD.0000000000003365. Medicine (Baltimore). 2016. PMID: 27082598 Free PMC article. - Tissue metabolism and host-microbial interactions in the intestinal mucosa.
Chun C, Zheng L, Colgan SP. Chun C, et al. Free Radic Biol Med. 2017 Apr;105:86-92. doi: 10.1016/j.freeradbiomed.2016.09.027. Epub 2016 Sep 28. Free Radic Biol Med. 2017. PMID: 27687211 Free PMC article. Review. - Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination.
Abbott RK, Thayer M, Labuda J, Silva M, Philbrook P, Cain DW, Kojima H, Hatfield S, Sethumadhavan S, Ohta A, Reinherz EL, Kelsoe G, Sitkovsky M. Abbott RK, et al. J Immunol. 2016 Nov 15;197(10):4014-4020. doi: 10.4049/jimmunol.1601401. Epub 2016 Oct 19. J Immunol. 2016. PMID: 27798169 Free PMC article. - Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment.
Graham K, Unger E. Graham K, et al. Int J Nanomedicine. 2018 Oct 4;13:6049-6058. doi: 10.2147/IJN.S140462. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30323592 Free PMC article. Review. - Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels.
Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, McKenna WG, Muschel RJ, Hiraoka M. Harada H, et al. Nat Commun. 2012 Apr 17;3:783. doi: 10.1038/ncomms1786. Nat Commun. 2012. PMID: 22510688 Free PMC article.
References
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Sem Radiat Oncol 2004; 14: 198–206. - PubMed
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4: 437–47. - PubMed
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. - PubMed
- Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical