Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells - PubMed (original) (raw)
Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells
David J Chung et al. Blood. 2009.
Abstract
A comprehensive understanding of the complex, autologous cellular interactions and regulatory mechanisms that occur during normal dendritic cell (DC)-stimulated immune responses is critical to optimizing DC-based immunotherapy. We have found that mature, immunogenic human monocyte-derived DCs (moDCs) up-regulate the immune-inhibitory enzyme, indoleamine 2,3-dioxygenase (IDO). Under stringent autologous culture conditions without exogenous cytokines, mature moDCs expand regulatory T cells (Tregs) by an IDO-dependent mechanism. The priming of resting T cells with autologous, IDO-expressing, mature moDCs results in up to 10-fold expansion of CD4(+)CD25(bright)Foxp3(+)CD127(neg) Tregs. Treg expansion requires moDC contact, CD80/CD86 ligation, and endogenous interleukin-2. Cytofluorographically sorted CD4(+) CD25(bright)Foxp3(+) Tregs inhibit as much as 80% to 90% of DC-stimulated autologous and allogeneic T-cell proliferation, in a dose-dependent manner at Treg:T-cell ratios of 1:1, 1:5, and as low as 1:25. CD4(+)CD25(bright)Foxp3(+) Tregs also suppress the generation of cytotoxic T lymphocytes specific for the Wilms tumor antigen 1, resulting in more than an 80% decrease in specific target cell lysis. Suppression by Tregs is both contact-dependent and transforming growth factor-beta-mediated. Although mature moDCs can generate Tregs by this IDO-dependent mechanism to limit otherwise unrestrained immune responses, inhibition of this counter-regulatory pathway should also prove useful in sustaining responses stimulated by DC-based immunotherapy.
Figures
Figure 1
IDO protein expression and activity increase with maturation of human moDCs. (A) IDO protein expression was assessed by flow cytometry in immature, CD83neg moDCs and in mature, CD83+ moDCs. Data are representative of 1 of 10 experiments. (B) IDO protein expression was assessed by Western blot in cell extracts from immature and mature moDCs. Untreated and IFN-γ–treated HeLa cells served as negative and positive controls, respectively. A representative blot from 1 experiment is shown along with pooled densitometry data from 4 separate experiments (mean ± SD; P = NS) showing relative IDO expression between groups. Densitometry values for each group were normalized to Rho-GDI (internal control). Relative IDO expression indicates fold increase above baseline IDO expression in untreated HeLa cells. (C) Supernatants from equal numbers of immature and mature moDCs were resuspended in tryptophan-enriched medium and tested after 4 hours by HPLC for production of the tryptophan catabolite, kynurenine, as an index of IDO activity (average of triplicate means ± SEM, n = 4 independent experiments). *P < .01 vs immature moDCs. (D) Kynurenine concentrations were determined by a spectrophotometric assay at 1 and 4 hours, using culture parameters identical to those in panel C (average of triplicate means ± SEM, n = 3 independent experiments). *P < .001 vs immature moDCs.
Figure 2
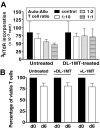
IDO-expressing, mature moDCs prime autologous T cells to suppress allogeneic T-cell responses. (A) T cells were cultured twice for 6 days, each with fresh autologous, mature moDCs, with or without the IDO inhibitor,
dl
-1MT. These primed T cells (Auto) from either the untreated or
dl
-1MT–treated group were harvested, washed, and added to separate allogeneic MLRs composed of fresh autologous moDCs with new allogeneic responder T cells (Allo), but no
dl
-1MT. MoDC to new allogeneic responder T-cell ratio was 1:30; and candidate suppressor T-cell (Auto) to responder allogeneic T-cell (Allo) ratios were 1:10 (□), 1:2 (▩), and 1:1 (▤). Controls were MLRs without suppressor cells (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of triplicate means ± SEM, n = 6 independent experiments; P = NS). (B) T-cell viability was determined by trypan blue exclusion and was compared in autologous priming cultures with or without
dl
-1MT or
l
-1MT. T-cell counts were determined at day 0 and after 6 days of culture (average of triplicate means ± SEM, n = 6 independent experiments; P = NS).
Figure 3
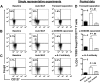
IDO-expressing, mature moDCs expand Foxp3+CD127neg Tregs and induce Tregs from CD4+CD25neg precursors in autologous culture. (A) Baseline expression of Foxp3 and CD127 by unprimed bulk CD4+ T cells was assessed by flow cytometry. T cells were cultured with or without the IDO inhibitor,
l
-1MT, and stimulated by autologous, mature moDCs for 6 days. The percentage of CD4+CD25+Foxp3+CD127neg Tregs among all CD4+ T cells was assessed by flow cytometry at day 6. (B) Pooled data from 5 independent experiments show the percentage of CD4+CD25+Foxp3+CD127neg Tregs at baseline before priming (d0) and after 6 days' stimulation by autologous, mature moDCs either without (□) or with (▩) [
l
-1MT (mean ± SEM, number of independent experiments, and P value indicated on graph). (C) Unprimed bulk T cells were sorted cytofluorographically to collect CD4+CD25neg T cells. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (day 0) and after 6 days' stimulation by autologous moDCs either with (▤) or without (▩)
l
-1MT. Controls were unmanipulated bulk T cells at day 0 (■) and after 6 days' stimulation by autologous moDCs (□; mean ± SEM; number of independent experiments and P value indicated on graph). (D) Representative histograms show percentages of Ki67-positive T cells in CD4+CD25low/intFoxpneg (Non-Tregs) and CD4+CD25brightFoxp+ (Tregs) cells from cultures without
l
-1MT, and in Tregs from
l
-1MT–treated cultures (mean ± SD; n = 2 independent experiments).
Figure 4
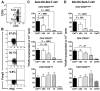
Expansion of Tregs by autologous, IDO-expressing, mature moDCs involves cell-to-cell contact, CD80/86 ligation, and IL-2. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (left panels), after 6 days' stimulation by autologous DCs (Auto MLR, middle panels), and after 6 days' stimulation by autologous moDCs (A) with transwell separation of moDCs from responder T cells (transwell separation), (B) after opsonization of moDCs with anti-CD80 and anti-CD86 a priori (α-CD80/86 opsonized), and (C) in the presence of neutralizing anti–IL-2 (α-IL-2 antibody; right panel for each condition). Isotype-matched, nonreactive antibodies were added to the control auto-MLRs in panels B and C. (D) Pooled data are shown for each experimental variable in rows A through C (mean ± SEM; number of independent experiments and P value indicated on each graph).
Figure 5
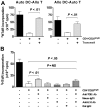
Suppression of both autologous and allogeneic T-cell responses segregates with the CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs. (A) T cells harvested after two 6-day rounds of priming with autologous, mature moDCs were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups, and (B) assessed for Foxp3 expression postsort. Data are from 1 representative experiment of 4. (C-D) Cytofluorographically sorted CD4+CD25bright, CD4+CD25int, and CD4+CD25neg T cells were added to allogeneic MLRs composed of either fresh autologous moDCs and new allogeneic T cells (C) or fresh autologous T cells with new allogeneic moDCs (D). MoDC to responder T-cell ratio was 1:30 throughout, and candidate suppressor T-cell to responder T-cell ratios were 1:25 (□), 1:5 (▩), and 1:1 (▤). Controls included MLRs without suppressor cells (Ctrl#1, ■) and moDCs cultured with candidate suppressor cells without responder T cells (Ctrl#2,  ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
Figure 6
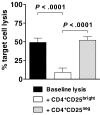
Inhibition of antigen-specific CTL generation by CD4+CD25brightFoxp3+ Tregs. Bulk T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright and CD4+CD25neg candidate Tregs. These were then added 1:1 with responder T cells to mature moDCs that were electroporated before terminal maturation with WT1 mRNA. After 7 days' culture, WT1-specific target cell lysis was measured by a flow cytometric PKH-26 cell lysis assay and compared between cultures containing no candidate Tregs (baseline lysis, ■), CD4+CD25bright T cells (□), and CD4+CD25neg T cells (▩; average of triplicate means ± SEM, n = 3 independent experiments; P values indicated on graph).
Figure 7
Suppression of T-cell proliferation by CD4+CD25brightFoxp3+ Tregs involves both contact-dependent and contact-independent mechanisms. (A) Autologous T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright candidate Tregs. These were then added 1:1 with either (A left panel) new allogeneic responder T cells stimulated by freshly thawed, mature moDCs, autologous to the candidate Tregs, or (A right panel) new responder T cells, autologous to the candidate Tregs, stimulated by new allogeneic, mature moDCs. The candidate Tregs were in contact (□) or separated in transwell inserts (▩) from the responder T cells, compared with control MLRs containing no candidate Tregs (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of quadruplicate means ± SEM, n = 2 independent experiments; P value indicated on each graph). (B) Neutralizing anti–TGF-β and anti–IL-10 antibodies, or their respective isotype-matched, nonreactive antibodies (mouse and rat IgG1), were added to MLRs containing CD4+CD25bright candidate Tregs added 1:1 with new allogeneic responder T cells stimulated by mature moDCs, autologous to the candidate Tregs. After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with control MLRs containing no candidate Tregs (■) and control MLRs containing candidate Tregs without neutralizing antibodies (□; average of triplicate means ± SEM, n = 2 independent experiments; P values indicated on graph).
Similar articles
- Indoleamine 2,3-dioxygenase-expressing leukemic dendritic cells impair a leukemia-specific immune response by inducing potent T regulatory cells.
Curti A, Trabanelli S, Onofri C, Aluigi M, Salvestrini V, Ocadlikova D, Evangelisti C, Rutella S, De Cristofaro R, Ottaviani E, Baccarani M, Lemoli RM. Curti A, et al. Haematologica. 2010 Dec;95(12):2022-30. doi: 10.3324/haematol.2010.025924. Epub 2010 Aug 26. Haematologica. 2010. PMID: 20801903 Free PMC article. - IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells.
Curran TA, Jalili RB, Farrokhi A, Ghahary A. Curran TA, et al. Immunobiology. 2014 Jan;219(1):17-24. doi: 10.1016/j.imbio.2013.06.008. Epub 2013 Jun 25. Immunobiology. 2014. PMID: 23891282 - Allogeneic Mature Human Dendritic Cells Generate Superior Alloreactive Regulatory T Cells in the Presence of IL-15.
Litjens NH, Boer K, Zuijderwijk JM, Klepper M, Peeters AM, Prens EP, Verschoor W, Kraaijeveld R, Ozgur Z, van den Hout-van Vroonhoven MC, van IJcken WF, Baan CC, Betjes MG. Litjens NH, et al. J Immunol. 2015 Jun 1;194(11):5282-93. doi: 10.4049/jimmunol.1402827. Epub 2015 Apr 27. J Immunol. 2015. PMID: 25917092 - T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.
Kahler DJ, Mellor AL. Kahler DJ, et al. Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review. - Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.
Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Yamazaki S, et al. Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review.
Cited by
- The Roles of Gut Microbiota Metabolites in the Occurrence and Development of Colorectal Cancer: Multiple Insights for Potential Clinical Applications.
Cheng W, Li F, Yang R. Cheng W, et al. Gastro Hep Adv. 2024 Jun 7;3(6):855-870. doi: 10.1016/j.gastha.2024.05.012. eCollection 2024. Gastro Hep Adv. 2024. PMID: 39280926 Free PMC article. Review. - Loss of DOCK2 potentiates Inflammatory Bowel Disease-associated colorectal cancer via immune dysfunction and IFNγ induction of IDO1 expression.
Churchhouse AMD, Billard CV, Suzuki T, Pohl SÖG, Doleschall NJ, Donnelly K, Nixon C, Arends MJ, Din S, Kirkwood K, Marques Junior J, Von Kriegsheim A, Coffelt SB, Myant KB. Churchhouse AMD, et al. Oncogene. 2024 Oct;43(42):3094-3107. doi: 10.1038/s41388-024-03135-9. Epub 2024 Sep 7. Oncogene. 2024. PMID: 39242821 Free PMC article. - A role of gut microbiota metabolites in HLA-E and NKG2 blockage immunotherapy against tumors: new insights for clinical application.
Cheng W, Zhu N, Wang J, Yang R. Cheng W, et al. Front Immunol. 2024 Aug 20;15:1331518. doi: 10.3389/fimmu.2024.1331518. eCollection 2024. Front Immunol. 2024. PMID: 39229258 Free PMC article. Review. - Interstitial Foci Expression of Indoleamine 2,3-Dioxygenase 1: A Potential Biomarker for Kidney Transplant Rejection.
Wiśnicki K, Donizy P, Kuriata-Kordek M, Uchmanowicz I, Zachciał J, Hałoń A, Janczak D, Banasik M. Wiśnicki K, et al. J Clin Med. 2024 Jul 22;13(14):4265. doi: 10.3390/jcm13144265. J Clin Med. 2024. PMID: 39064305 Free PMC article. - Effects of the gut microbiota and its metabolite short-chain fatty acids on endometriosis.
Liu M, Peng R, Tian C, Shi J, Ma J, Shi R, Qi X, Zhao R, Guan H. Liu M, et al. Front Cell Infect Microbiol. 2024 Jun 13;14:1373004. doi: 10.3389/fcimb.2024.1373004. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38938880 Free PMC article. Review.
References
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
- Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. - PubMed
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA023766/CA/NCI NIH HHS/United States
- R01 CA083070/CA/NCI NIH HHS/United States
- R01 CA118974/CA/NCI NIH HHS/United States
- P01 CA23766/CA/NCI NIH HHS/United States
- R01 CA112431/CA/NCI NIH HHS/United States
- R01 CA83070/CA/NCI NIH HHS/United States
- R21 CA119528/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials