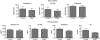Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease - PubMed (original) (raw)
Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease
Yinxing Liu et al. Mol Ther. 2009 Aug.
Abstract
Neprilysin (NEP) is a zinc metallopeptidase that efficiently degrades the amyloid beta (Abeta) peptides believed to be involved in the etiology of Alzheimer disease (AD). The focus of this study was to develop a new and tractable therapeutic approach for treating AD using NEP gene therapy. We have introduced adeno-associated virus (AAV) expressing the mouse NEP gene into the hindlimb muscle of 6-month-old human amyloid precursor protein (hAPP) (3X-Tg-AD) mice, an age which correlates with early stage AD. Overexpression of NEP in muscle decreased brain soluble Abeta peptide levels by approximately 60% and decreased amyloid deposits by approximately 50%, with no apparent adverse effects. Expression of NEP on muscle did not affect the levels of a number of other physiological peptides known to be in vitro substrates. These findings demonstrate that peripheral expression of NEP and likely other peptidases represents an alternative to direct administration into brain and illustrates the potential for using NEP expression in muscle for the prevention and treatment of AD.
Figures
**Figure 1
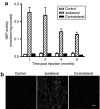
Expression of mouse NEP in mouse limb muscle is dependent on the dose of NEP-AAV8. Nine-month-old 3X-Tg-AD mice were injected with the indicated amount of NEP-AAV8 viral genomes (vg) into the hindlimb. Injections were 5 µl per site using six sites in the hindlimb muscle. Six weeks post injection, the mice were killed, the muscle dissected, and homogenates prepared in phosphate-buffered saline. Western blot analysis was performed using 50 µg of muscle homogenate and goat anti-mouse neprilysin. C, untreated 3X-Tg-AD mice; NEP, neprilysin; sNEP, 50 ng of purified NEP.
**Figure 2
NEP expression in muscle mediated by AAV8 is sustained post injection. (a) NEP activity in muscle 4 months post injection of NEP-AAV8. Nine-month-old 3X-Tg-AD mice were injected with 2 × 1011 viral genomes (vg) of either mNEP-AAV8 or GFP-AAV8 into six sites within the hindlimb (5 µl per site), with the contralateral hindlimb untreated. Mice were killed at the indicated times post injection, the muscle dissected out, and used to measure NEP activity. Data are presented as mean ± SEM. (n = 3). (b) Immunohistochemistry of NEP in muscle. Sections of muscle from mice at 4 months post injection with 2 × 1011 vg NEP-AAV8 were stained with anti-mouse NEP antibody. Scale bar presents 100 µm. NEP, neprilysin.
**Figure 3
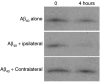
Neprilysin (NEP) expressed on muscle is active toward degrading Aβ. Aβ1–40 (2 µmol/l) was incubated with muscle homogenates (2.2–2.3 µg protein) for 4 hours in 100 µl phosphate-buffered saline buffer. Samples were then run on an SDS-Tricine gel and Aβ1–40 detected by western blot analysis with monoclonal antibody 6E10. Note, in the ipsilateral muscle expressing NEP, the Aβ is mostly cleaved, whereas no detectable cleavage was observed with the control (contralateral) muscle homogenates.
**Figure 4
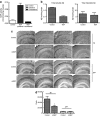
Effect of hindlimb muscle NEP expression on brain Aβ. At ~6 months of age, 3X-Tg-AD mice (n = 7) were injected in one hindlimb muscle with 2 × 1011 viral genomes (vg) of NEP-AAV8. When the mice reached 12 months of age, they were killed and analyzed for NEP expression and Aβ levels. (a) NEP expression in mouse hindlimb 6 months post injection. Hindlimb muscle was homogenized in phosphate-buffered saline and used to measure NEP activity as in Figure 1. Data are presented as mean ± SEM. (n = 7). (b) Aβ in mouse brain 6 months post intramuscular injection of NEP-AAV8. Aβ levels in 3X-Tg-AD mouse brain 6 months following intramuscular injection of NEP-AAV8 (2 × 1011 vg) decreased as determined by sandwich enzyme-linked immunosorbent assay. Data are presented as mean ± SEM. (n = 7). Comparing the means of NEP-treated versus control mice for Aβ, the P value is 0.0025. Due to multiple testing, a Bonferroni correction was applied and a significance level of 0.025 was used. By this criteria, the lower brain Aβ retained its statistical significance in the NEP-expressing mice. (c) Aβ and hAPP immunohistochemistry in the brain of mice expressing hindlimb NEP. Brain sections were stained with anti-Aβ42 and anti-APP–specific antibodies and developed using DAB. Aβ42 and APP were measured in adjacent sections of cortex and hippocampus, n = 7. Scale bar is 500 µm. (d) Quantitative evaluation of Aβ deposits in hippocampal and cortical regions. The data from c was quantified as described in Materials and Methods. NEP-expressing mice show reduced Aβ deposits versus control green fluorescent protein–expressing mice, *P < 0.05, n = 7. APP, amyloid precursor protein; mNEP, mouse neprilysin.
**Figure 5
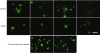
Thioflavine S staining of plaques in brain hippocampus. Mouse brains were stained with thioflavine S as described in Materials and Methods. Few mature plaques are seen in the 12-month-old 3XTg-AD mouse brain compared to a 17-month-old 3XTg-AD mouse brain, and little difference between NEP-treated and -untreated mice was observed. mNEP, mouse neprilysin. Bar = 50 µm.
**Figure 6
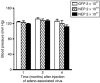
NEP expression in the mouse hindlimb does not affect blood pressure. Nine-month-old 3X-Tg-AD mice were injected with the indicated number of viral genomes of either mNEP-AAV8 or GFP-AAV8 into hindlimb as described in Figure 3. Systolic blood pressure was measured as described in Materials and Methods at 1, 2, and 4 months post injection. Data are presented as mean ± SEM (n = 9 for 2 × 1011 viral genomes of AAV-GFP and NEP, n = 5 for 2 × 1010 vg of AAV-NEP). No statistically significant difference between any groups was noted. GFP, green fluorescent protein; NEP, neprilysin.
**Figure 7
NEP expression in the mouse hindlimb does not affect the level of substance P, leucine-enkephalin, and bradykinin in brain or blood. Commercial ELISA kits were used to measure substance P, bradykinin, and leucine-enkephalin in brain or plasma samples prepared as described in Materials and Methods. Plasma Aβ levels were measured as described in Methods. No statistically significant difference between any groups was noted. Leu-Enk, leucine-enkephalin; mNEP, mouse neprilysin.
Similar articles
- Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease.
Hüttenrauch M, Baches S, Gerth J, Bayer TA, Weggen S, Wirths O. Hüttenrauch M, et al. J Alzheimers Dis. 2015;44(4):1291-302. doi: 10.3233/JAD-142463. J Alzheimers Dis. 2015. PMID: 25408216 - Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice.
Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L. Meilandt WJ, et al. J Neurosci. 2009 Feb 18;29(7):1977-86. doi: 10.1523/JNEUROSCI.2984-08.2009. J Neurosci. 2009. PMID: 19228952 Free PMC article. - Activating AhR alleviates cognitive deficits of Alzheimer's disease model mice by upregulating endogenous Aβ catabolic enzyme Neprilysin.
Qian C, Yang C, Lu M, Bao J, Shen H, Deng B, Li S, Li W, Zhang M, Cao C. Qian C, et al. Theranostics. 2021 Aug 11;11(18):8797-8812. doi: 10.7150/thno.61601. eCollection 2021. Theranostics. 2021. PMID: 34522212 Free PMC article. - NEP-like endopeptidases and Alzheimer's disease [corrected].
Marr RA, Spencer BJ. Marr RA, et al. Curr Alzheimer Res. 2010 May;7(3):223-9. doi: 10.2174/156720510791050849. Curr Alzheimer Res. 2010. PMID: 20088804 Review. - [Elucidating Pathogenic Mechanisms of Early-onset Alzheimer's Disease in Down Syndrome Patients].
Asai M, Kawakubo T, Mori R, Iwata N. Asai M, et al. Yakugaku Zasshi. 2017;137(7):801-805. doi: 10.1248/yakushi.16-00236-2. Yakugaku Zasshi. 2017. PMID: 28674290 Review. Japanese.
Cited by
- Neprilysin expression and functions in development, ageing and disease.
Nalivaeva NN, Zhuravin IA, Turner AJ. Nalivaeva NN, et al. Mech Ageing Dev. 2020 Dec;192:111363. doi: 10.1016/j.mad.2020.111363. Epub 2020 Sep 26. Mech Ageing Dev. 2020. PMID: 32987038 Free PMC article. Review. - Neprilysin-2 is an important β-amyloid degrading enzyme.
Hafez D, Huang JY, Huynh AM, Valtierra S, Rockenstein E, Bruno AM, Lu B, DesGroseillers L, Masliah E, Marr RA. Hafez D, et al. Am J Pathol. 2011 Jan;178(1):306-12. doi: 10.1016/j.ajpath.2010.11.012. Epub 2010 Dec 23. Am J Pathol. 2011. PMID: 21224067 Free PMC article. - Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders.
Zlokovic BV. Zlokovic BV. Nat Rev Neurosci. 2011 Nov 3;12(12):723-38. doi: 10.1038/nrn3114. Nat Rev Neurosci. 2011. PMID: 22048062 Free PMC article. Review. - Catalytic immunoglobulin gene delivery in a mouse model of Alzheimer's disease: prophylactic and therapeutic applications.
Kou J, Yang J, Lim JE, Pattanayak A, Song M, Planque S, Paul S, Fukuchi K. Kou J, et al. Mol Neurobiol. 2015 Feb;51(1):43-56. doi: 10.1007/s12035-014-8691-z. Epub 2014 Apr 15. Mol Neurobiol. 2015. PMID: 24733587 Free PMC article. - Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer's disease.
Nalivaeva NN, Belyaev ND, Kerridge C, Turner AJ. Nalivaeva NN, et al. Front Aging Neurosci. 2014 Sep 17;6:235. doi: 10.3389/fnagi.2014.00235. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25278875 Free PMC article. Review.
References
- Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer's disease. Annu Rev Cell Biol. 1994;10:373–403. - PubMed
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, et al. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. - PubMed
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20RR02017/RR/NCRR NIH HHS/United States
- AG 24899/AG/NIA NIH HHS/United States
- R01 DA002243/DA/NIDA NIH HHS/United States
- DA 02243/DA/NIDA NIH HHS/United States
- R21 AG024899/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous