Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict - PubMed (original) (raw)
Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict
Rogier B Mars et al. J Neurosci. 2009.
Abstract
Medial frontal cortex (MFC) is crucial when actions have to be inhibited, reprogrammed, or selected under conflict, but the precise mechanism by which it operates is unclear. Importantly, how and when the MFC influences the primary motor cortex (M1) during action selection is unknown. Using paired-pulse transcranial magnetic stimulation, we investigated functional connectivity between the presupplementary motor area (pre-SMA) part of MFC and M1. We found that functional connectivity increased in a manner dependent on cognitive context: pre-SMA facilitated the motor evoked-potential elicited by M1 stimulation only during action reprogramming, but not when otherwise identical actions were made in the absence of conflict. The effect was anatomically specific to pre-SMA; it was not seen when adjacent brain regions were stimulated. We discuss implications for the anatomical pathways mediating the observed effects.
Figures
Figure 1.
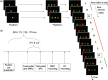
a, On each trial of the action reprogramming task participants were presented with a centrally displayed white square. Subsequently, two colored flankers (red and green, sides random) appeared on either side of fixation. Four hundred and fifty to six hundred milliseconds after flanker onset, a central colored cue appeared, to which participants responded with the index finger of the hand on the side with the congruent color. Trials were blocked into groups with the same cue color, so that as soon as flankers were presented, participants could anticipate and thus prepare an action based on the cue color presented in the previous trial. The prepared response would, however, be incorrect when the central cue color changed from one trial to the next (switch trials, boxed letters). Correct actions are indicated by “R” (right) and “L” (left). b, The M1 test pulse was applied 75, 125, or 175 ms after the central color cue onset. A pre-SMA conditioning pulse preceded the M1 test pulse by 6 ms on half of the TMS trials.
Figure 2.
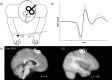
a, In the switch and stay experiments, the test coil (black) was placed over left M1, whereas the conditioning coil (white) was placed over pre-SMA. b, Example of MEPs recorded on a single pulse (black) and a dual pulse (gray) trial. The conditioning pulse can modulate peak-to-peak MEP amplitude. c, Sagittal views of the mean anatomical image indicating pre-SMA (left) and M1 (right) TMS sites. Each circle represents the coil location in one participant.
Figure 3.
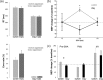
a, Behavioral data in the main switch and stay experiments indicate participants reported faster (top) and more accurate (bottom) on switch compared with stay trials. b, The effect of pre-SMA conditioning pulses on M1 test pulse-elicited MEP amplitudes was specific to behavioral context and SOA. Pre-SMA/M1 functional connectivity significantly increased on switch trials. * indicates significant modulation of MEP amplitudes in dual-pulse compared with respective single-pulse trials. c, Context-specific facilitations of MEP amplitude at a SOA of 125 ms were only present when the conditioning coil was place over pre-SMA and were absent when the conditioning coil was over PMd or M1.
Figure 4.
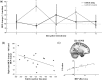
Additional results. a, Significant MEP facilitation was seen when switching toward the contralateral hand at 6 and 12 ms interpulse intervals, but no significant effect was seen when switching to the ipsilateral hand. b, A negative correlation was present between the relative facilitation of the contralateral, compared with the ipsilateral, hand and the RT on switch trials. c, Example location of significant correlation between MEP effect size and white matter intensity and scatter plot of individual data within this cluster.
Similar articles
- Cortical and subcortical interactions during action reprogramming and their related white matter pathways.
Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Neubert FX, et al. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13240-5. doi: 10.1073/pnas.1000674107. Epub 2010 Jul 9. Proc Natl Acad Sci U S A. 2010. PMID: 20622155 Free PMC article. Clinical Trial. - Top-down inhibitory control exerted by the medial frontal cortex during action selection under conflict.
Duque J, Olivier E, Rushworth M. Duque J, et al. J Cogn Neurosci. 2013 Oct;25(10):1634-48. doi: 10.1162/jocn_a_00421. Epub 2013 May 10. J Cogn Neurosci. 2013. PMID: 23662862 - Functional specificity of human premotor-motor cortical interactions during action selection.
O'Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. O'Shea J, et al. Eur J Neurosci. 2007 Oct;26(7):2085-95. doi: 10.1111/j.1460-9568.2007.05795.x. Epub 2007 Sep 14. Eur J Neurosci. 2007. PMID: 17868374 - Increased primary motor cortical excitability by a single-pulse transcranial magnetic stimulation over the supplementary motor area.
Shirota Y, Hamada M, Terao Y, Ohminami S, Tsutsumi R, Ugawa Y, Hanajima R. Shirota Y, et al. Exp Brain Res. 2012 Jun;219(3):339-49. doi: 10.1007/s00221-012-3095-7. Epub 2012 Apr 25. Exp Brain Res. 2012. PMID: 22532164 - Intention, choice, and the medial frontal cortex.
Rushworth MF. Rushworth MF. Ann N Y Acad Sci. 2008 Mar;1124:181-207. doi: 10.1196/annals.1440.014. Ann N Y Acad Sci. 2008. PMID: 18400931 Review.
Cited by
- Manual action re-planning interferes with the maintenance process of working memory: an ERP investigation.
Gunduz Can R, Schack T, Koester D. Gunduz Can R, et al. Psychol Res. 2023 Sep;87(6):1784-1805. doi: 10.1007/s00426-022-01741-4. Epub 2022 Nov 24. Psychol Res. 2023. PMID: 36434433 Free PMC article. - Anodal tDCS modulates specific processing codes during conflict monitoring associated with superior and middle frontal cortices.
Adelhöfer N, Stock AK, Beste C. Adelhöfer N, et al. Brain Struct Funct. 2021 May;226(4):1335-1351. doi: 10.1007/s00429-021-02245-4. Epub 2021 Mar 3. Brain Struct Funct. 2021. PMID: 33656578 Free PMC article. - Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency.
Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Moore JW, et al. Proc Biol Sci. 2010 Aug 22;277(1693):2503-9. doi: 10.1098/rspb.2010.0404. Epub 2010 Apr 7. Proc Biol Sci. 2010. PMID: 20375048 Free PMC article. - Overcoming status quo bias in the human brain.
Fleming SM, Thomas CL, Dolan RJ. Fleming SM, et al. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):6005-9. doi: 10.1073/pnas.0910380107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231462 Free PMC article. - Transcranial Magnetic Mapping of the Short-Latency Modulations of Corticospinal Activity from the Ipsilateral Hemisphere during Rest.
Cattaneo L, Barchiesi G. Cattaneo L, et al. Front Neural Circuits. 2011 Oct 18;5:14. doi: 10.3389/fncir.2011.00014. eCollection 2011. Front Neural Circuits. 2011. PMID: 22022307 Free PMC article.
References
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. - PubMed
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. - PubMed
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources