Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy - PubMed (original) (raw)
Clinical Trial
Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy
L A Cysique et al. Neurology. 2009.
Abstract
Objective: To rigorously evaluate the time course of cognitive change in a cohort of individuals with HIV-associated neurocognitive disorders (HAND) initiating combination antiretroviral therapy (CART), and to investigate which demographic, laboratory, and treatment factors are associated with neuropsychological (NP) outcome (or "any NP improvement").
Methods: Study participants included 37 HIV+ individuals with mild to moderate NP impairment who initiated CART and underwent NP testing at 12, 24, 36, and 48 weeks thereafter. NP change was assessed using a regression-based change score that was normed on a separate NP-stable group thereby controlling for regression toward the mean and practice effect. Mixed-effect regression models adjusting for loss to follow-up were used to evaluate the time course of cognitive change and its association with baseline and time-varying predictors.
Results: In persons with HAND initiating CART, cognitive improvement happens soon after initiation (13% at week 12), but more often 24, 36, and up to 48 weeks after initiation (up to 41%), with fewer than 5% demonstrating significant worsening. In multivariate analyses, unique predictors of NP improvement included more severe baseline NP impairment and higher CART CNS penetration index. Greater viral load decrease was associated with NP improvement only in univariate analyses.
Conclusion: Clinically meaningful neuropsychological improvement seemed to peak around 24-36 weeks after combination antiretroviral therapy initiation and was prolonged over the 1-year study period. This study also provides new evidence that benefit may be maximized by choosing antiretroviral medications that reach therapeutic concentrations in the CNS.
Figures
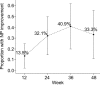
Figure 1 Proportion of HIV+ individuals with neuropsychological improvement from baseline Proportion of participants with neuropsychological (NP) improvement at each session was derived from the categorical mean scaled score regression change score (MS-Reg-CS). The MS-Reg-CS provides a standard score that can be used as a continuous score or a categorical score (i.e., significant NP improvement as MS-Reg-CS ≥1.645 and NP decline as MS-Reg-CS ≤−1.645, based on a two-tailed 90% confidence interval; individuals within these ranges were classified as NP stable). Only one individual declined at week 36 (4.5%). In this figure, 95% confidence intervals are provided around the observed proportions of NP change at each visit. At each study time point, proportion of improving cases is different from zero.
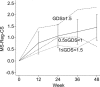
Figure 2 Average change in neuropsychological performance across study time MS-Reg-CS = mean scaled score regression change score. A negative MS-Reg-CS indicates decline, while a positive MS-Reg-CS indicates improvement. Significant decline was defined as a MS-Reg-CS ≥−1.645 and improvement was defined as a MS-Reg-CS ≥+1.645, which is equivalent to a 90% confidence interval. Observed standard deviation for MS-Reg-CS: week 12 = 1.19; week 24 = 1.37; week 36 = 2.00; week 48 = 1.83. Mixed effect regression model (4*) showed that change in the MS-Reg-CS was significant at visit 12 (p = 0.002) as compared to baseline. Subsequent MS-Reg-CS at week 36 (p = 0.019) and week 48 (p = 0.003) visits were significantly different compared to week 12 visit, but not at week 24 visit (p = 0.11). *Wald test of random intercept model. A higher GDS represents lower performance. We illustrated three profiles of improvement depending on three different levels of baseline impairment (mild impairment: 0.5 ≤ GDS < 1; moderate impairment: 1 ≤ GDS <1.5; and severe impairment: GDS ≥1.5). Only the 37 patients’ average MS-Reg-CS was tested. In other words, the impairment subgroups were not statistically compared due to sample sizes and resulting power issues. To explore the advantage of using the MS-Reg-CS as the outcome variable, we conducted the same statistical analyses simply using the uncorrected mean scaled scores. We found NP improvement to be highly significant at all time points (p < 0.0001), which differs substantially from the above p values, reflecting an inflated improvement probably corresponding to uncorrected regression toward the mean and practice effect. Note that since the response measures change from baseline, the week 0 responses are not used in the model.
Comment in
- HIV-associated brain dysfunction in the era of HAART: reasons for hope, but continued concern.
Cohen RA, Gongvatana A. Cohen RA, et al. Neurology. 2009 Aug 4;73(5):338-9. doi: 10.1212/WNL.0b013e3181b1220d. Epub 2009 Jun 24. Neurology. 2009. PMID: 19553591 No abstract available.
Similar articles
- HIV-associated brain dysfunction in the era of HAART: reasons for hope, but continued concern.
Cohen RA, Gongvatana A. Cohen RA, et al. Neurology. 2009 Aug 4;73(5):338-9. doi: 10.1212/WNL.0b013e3181b1220d. Epub 2009 Jun 24. Neurology. 2009. PMID: 19553591 No abstract available. - Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda.
Sacktor N, Nakasujja N, Skolasky RL, Robertson K, Musisi S, Ronald A, Katabira E, Clifford DB. Sacktor N, et al. Neurology. 2009 Jan 13;72(2):165-70. doi: 10.1212/01.wnl.0000339042.96109.86. Neurology. 2009. PMID: 19139369 Free PMC article. Clinical Trial. - Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND).
Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N, Nakamoto B, Shiramizu BT, Shikuma CM. Ndhlovu LC, et al. J Neurovirol. 2014 Dec;20(6):571-82. doi: 10.1007/s13365-014-0279-x. Epub 2014 Sep 17. J Neurovirol. 2014. PMID: 25227930 Free PMC article. - Dementia and neurocognitive disorders due to HIV-1 infection.
Ances BM, Ellis RJ. Ances BM, et al. Semin Neurol. 2007 Feb;27(1):86-92. doi: 10.1055/s-2006-956759. Semin Neurol. 2007. PMID: 17226745 Review. - CSF penetration by antiretroviral drugs.
Eisfeld C, Reichelt D, Evers S, Husstedt I. Eisfeld C, et al. CNS Drugs. 2013 Jan;27(1):31-55. doi: 10.1007/s40263-012-0018-x. CNS Drugs. 2013. PMID: 23160938 Review.
Cited by
- Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis.
Williams DW, Eugenin EA, Calderon TM, Berman JW. Williams DW, et al. J Leukoc Biol. 2012 Mar;91(3):401-15. doi: 10.1189/jlb.0811394. Epub 2012 Jan 6. J Leukoc Biol. 2012. PMID: 22227964 Free PMC article. Review. - Therapeutic amprenavir concentrations in cerebrospinal fluid.
Croteau D, Letendre S, Best BM, Rossi SS, Ellis RJ, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur J, McCutchan JA, Morgello S, Simpson DM, Way L, Capparelli E, Grant I; CHARTER Group. Croteau D, et al. Antimicrob Agents Chemother. 2012 Apr;56(4):1985-9. doi: 10.1128/AAC.05098-11. Epub 2012 Jan 30. Antimicrob Agents Chemother. 2012. PMID: 22290964 Free PMC article. - Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes.
Carvallo L, Lopez L, Che FY, Lim J, Eugenin EA, Williams DW, Nieves E, Calderon TM, Madrid-Aliste C, Fiser A, Weiss L, Angeletti RH, Berman JW. Carvallo L, et al. J Immunol. 2015 Apr 1;194(7):3246-58. doi: 10.4049/jimmunol.1302647. Epub 2015 Feb 25. J Immunol. 2015. PMID: 25716997 Free PMC article. - Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1.
Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D, Collier A, Gelman B, Marra C, Mbeo G, McCutchan A, Morgello S, Simpson D, Way L, Vaida F, Ueland S, Capparelli E, Grant I; CHARTER Group. Croteau D, et al. Antimicrob Agents Chemother. 2010 Dec;54(12):5156-60. doi: 10.1128/AAC.00507-10. Epub 2010 Sep 27. Antimicrob Agents Chemother. 2010. PMID: 20876368 Free PMC article. - Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change.
Cysique LA, Franklin D Jr, Abramson I, Ellis RJ, Letendre S, Collier A, Clifford D, Gelman B, McArthur J, Morgello S, Simpson D, McCutchan JA, Grant I, Heaton RK; CHARTER Group; HNRC Group. Cysique LA, et al. J Clin Exp Neuropsychol. 2011 Jun;33(5):505-22. doi: 10.1080/13803395.2010.535504. Epub 2011 Mar 7. J Clin Exp Neuropsychol. 2011. PMID: 21391011 Free PMC article.
References
- Sacktor N, Skolasky RL, Tarwater PM, et al. Response to systemic HIV viral load suppression correlates with psychomotor speed performance. Neurology 2003;61:567–569. - PubMed
- Roberston K, Roberston TW, Ford S, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr 2004;36:562–566. - PubMed
- Letendre S, McCutchan J, Childers M, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol 2004;56:416–423. - PubMed
- Ferrando SJ, Rabkin JG, van Gorp WG, Lin S-H, McElhiney M. Longitudinal improvement in psychomotor processing is associated with potent antiretroviral therapy in HIV-1 infection. J Neuropsychiatry Clin Neurosci 2003;15:208–214. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous