A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast - PubMed (original) (raw)
A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast
Liat Yakir-Tamang et al. Mol Biol Cell. 2009 Aug.
Abstract
The actin cytoskeleton rapidly depolarizes in yeast secretory (sec) mutants at restrictive temperatures. Thus, an unknown signal conferred upon secretion is necessary for actin polarity and exocytosis. Here, we show that a phosphatidylinositol (PI) transfer protein, Sfh5, and a phosphatidylinositol-4-phosphate 5-kinase, Mss4, facilitate Cdc42 activation to concomitantly regulate both actin and protein trafficking. Defects in Mss4 function led to actin depolarization, an inhibition of secretion, reduced levels of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P(2)] in membranes, mislocalization of a pleckstrin homology domain fused to green fluorescent protein, and the mislocalization of Cdc42. Similar defects were observed in sec, myo2-66, and cdc42-6 mutants at elevated temperatures and were rescued by the overexpression of MSS4. Likewise, the overexpression of SFH5 or CDC42 could ameliorate these defects in many sec mutants, most notably in sec3Delta cells, indicating that Cdc42-mediated effects upon actin and secretion do not necessitate Sec3 function. Moreover, mutation of the residues involved in PI binding in Sfh5 led to the mislocalization and loss of function of both Sfh5 and Cdc42. Based upon these findings, we propose that the exocytic signal involves PI delivery to the PI kinases (i.e., Mss4) by Sfh5, generation of PI(4,5)P(2), and PI(4,5)P(2)-dependent regulation of Cdc42 and the actin cytoskeleton.
Figures
Figure 1.
MSS4 overexpression rescues growth and secretion defects. (A) MSS4 overexpression suppresses growth defects. Indicated strains were transformed with control vector or vector overexpressing MSS4 (pRS426Mss4). Cells were grown overnight, diluted serially, plated, and incubated for 48 h at the indicated temperatures (Celsius). (B) A mss4-102 × sec8-9 cross leads to synthetic lethality. _MAT_α mss4-102 cells were crossed to _MAT_a sec8-9 cells. Meiotic segregants were tested for growth at 26 and 37°C on YPD. Spores that germinated but did not form a colony are denoted with an “x” (lethality). Viable colonies were tested for ts growth and presence of the _LEU2_-linked mss4-102 allele (Leu+). WT indicates colonies that were not ts. (C) Mutations with defects in secretion or actin are synthetic lethal with mss4-102. Indicated mutants were crossed with mss4-102 cells. Viability of the meiotic segregants and distribution of the ts and Leu+ (LEU2) markers were examined. “+” indicates viability, whereas “lethal” indicates inviability of the double mutants on YPD at 26°C. (D) mss4-102 cells are defective in invertase secretion and MSS4 overexpression restores secretion in sec12-1 cells. Invertase activity was measured in WT cells, sec12-1 cells transformed with empty vector or pRS426Mss4, and mss4-102 cells (lacking SUC2) bearing a plasmid expressing SUC2 from a starvation-inducible promoter (pUG36Suc2). sec12-1 and mss4-102 cells were derepressed (2.5 h) in low-glucose medium or low-glucose medium lacking methionine, respectively. Secretion index represents the percentage of external (secreted) invertase compared with total invertase activity measured at 26 and 37°C. The representative experiment shown plots the mean of triplicate samples for each strain assayed, along with an error bar representing the SE of the mean. This experiment was repeated two additional times and gave similar results (our unpublished observations).
Figure 2.
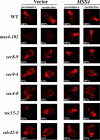
Actin delocalization in late sec mutants is rescued by MSS4 overexpression. Indicated strains transformed with control vector or pRS426Mss4 were grown to log phase and either maintained at 26°C (permissive) or shifted to elevated temperatures (restrictive) for 1 h before fixation and phalloidin labeling. The restrictive temperature was 33°C for cdc42-6 cells, whereas 37°C was used for other strains. Numbers indicate the percentage of cells, out of 100 cells examined (n = 100) for each strain, in which polarized actin was observed.
Figure 3.
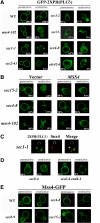
MSS4 overexpression restores PI(4,5)P2 distribution in sec mutants. (A) PI(4,5)P2 distribution is altered in the sec and cdc42-6 mutants. The indicated strains were transformed with a vector expressing GFP-2XPH(PLCδ) (pRS426GFP-2XPH(PLCδ)) and maintained at 26°C or shifted for 1 h at 37°C. Numbers indicate the percentage of cells in which PM localization of GFP-2XPH(PLCδ) was observed (n = 100 cells counted). (B) MSS4 overexpression restores normal PI(4,5)P2 distribution to the PM. The indicated strains were cotransformed with pRS426GFP-2XPH(PLCδ) and either a control vector or vector expressing MSS4 (pAD54Mss4). Cells were maintained at 26°C or shifted for 1 h to 37°C before visualization. Numbers indicate the percentage of cells in which PM localization of GFP-2XPH(PLCδ) was observed (n = 100 cells counted). (C) PI(4,5)P2 accumulates in endosomes upon blockage of the secretory pathway. sec1-1 cells expressing GFP-2XPH(PLCδ) and RFP-Snx4 (pAD54-RFP-Snx4) were grown at 26°C and shifted for 1 h to 37°C, before visualization. (D) PI(4,5)P2 accumulation in endosomes is dependent on endocytosis. sec6-4 and sec6-4 end4-1 cells expressing GFP-2XPH(PLCδ) were grown at 26°C and shifted for 1 h to 37°C, before visualization. (E) Mss4 localization is not altered in late sec mutants. Indicated strains were transformed with pUG35Mss4-GFP. Cells were induced in medium lacking methionine for 1 h and maintained at 26°C or shifted to 37°C for 1 h before visualization.
Figure 4.
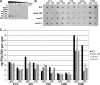
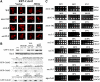
PI(4,5)P2 levels are altered in mss4-102 and sec mutants. (A) Specificity of anti-PIP2 antibodies. An array containing decreasing concentrations of phosphoinositide standards, as indicated, was probed with an anti-PIP2 antibody (1:5000). (B) Subcellular fractionation of PI(4,5)P2. The indicated strains were grown to mid-log phase and either maintained at 26°C or shifted for 1 h to 37°C and processed for cell fractionation (see Materials and Methods). Samples (0.5 μg of protein) from all fractions (S10, P10, S100, and P100) and TCLs were subjected to dot-blot analysis by using the anti-PIP2 antibody (1:5000). (C) Quantification of PI(4,5)P2. Densitometry was used to quantify PI(4,5)P2 in the different fractions, relative to the standard curve generated from A. Optical densities were measured and normalized for background, by using Image-Gauge software (Fujifilm). The amount of PIP2 present per 0.5 μg of protein for each fraction is graphed. A representative experiment out of three individual experiments having similar results is shown.
Figure 5.
Cdc42 localization is impaired in sec mutants and restored by MSS4 overexpression. (A) RFP-Cdc42 is mislocalized in the mss4-102 and sec mutants. The indicated strains were cotransformed with pAD54-RFP-Cdc42, which expresses RFP-Cdc42, and either a control vector or pRS426Mss4. Cells were maintained at 26°C or shifted for 1 h to 37°C before visualization. Numbers indicate the percentage of cells in which normal PM and vacuolar localization of RFP-Cdc42 was observed (n = 100 cells counted). (B) Subcellular fractionation of GFP-Cdc42. WT, mss4-102, and sec4-8 cells cotransformed with GFP-Cdc42 (pUG36GFP-Cdc42) and either a control vector or pGBKMss4 (+MSS4) and were grown to mid-log phase in medium lacking methionine. Cells were either maintained at 26°C or shifted for 1.5 h to 35°C and then lysed. Lysates were subjected to differential centrifugation to obtain the P10, S10, P100, and S100 fractions (see Materials and Methods). Samples of the fractions and TCLs were resolved by SDS-PAGE and detected in blots using anti-GFP and -Sso1 antibodies. (C) CDC42 overexpression enhances the growth of mss4-102, cdc42-6, and late sec mutants. mss4-102 cells were transformed either with control vector or pRS426Mss4 (uppermost row). Other mss4-102 cells were transformed with control vector or pUG36GFP-Cdc42 (third row from top). cdc42-6 cells were transformed with control vector or pRS426Mss4 (second row from top). Late sec mutants sec1-1, sec2-41, _sec3_Δ, sec4-8, sec15-2, and myo2-66 were transformed with control vector or pUG36GFP-Cdc42. Cells were grown in selective medium or medium lacking methionine, diluted serially, plated, and incubated for 48 h at the indicated temperatures (Celsius).
Figure 6.
Sfh5 acts with Mss4 to transduce the exocytic signal to Cdc42. (A) SFH5 overexpression rescues late sec mutants. Top, sec8-9 cells were transformed with control vector or pRS426Mss4. Second to fourth panels, sec8-9, sec2-41, and _sec3_Δ cells were transformed either with control vector or pRS426Sfh5 (SFH5). Cells were grown overnight, diluted serially, plated, and incubated for 48 h at the indicated temperatures (Celsius). (B) RFP-Cdc42 mislocalization in sec8-9 cells is rescued by SFH5 overexpression. The indicated strains were cotransformed with pAD54-RFP-Cdc42 and control vector, pRS426Mss4 (MSS4), or pRS426Sfh5 (SFH5). Cells were maintained at 26°C or shifted for 1 h to 37°C before visualization. (C) SFH5 overexpression enhances the growth of mss4-102 cells. mss4-102 cells were transformed with control vector, pUG35Mss4-GFP, or pUG36GFP-Sfh5. Cells were grown in medium lacking methionine, diluted serially, plated, and incubated for 48 h at the indicated temperatures (Celsius). (D) GFP-Sfh5 is mislocalized in sec mutants. The indicated strains were transformed with pUG36GFP-Sfh5, grown in medium lacking methionine (1 h), and either maintained at 26°C or shifted for 1 h to 37°C, before visualization. Numbers indicate the percentage of cells in which PM localization of GFP-Sfh5 was observed (n = 100 cells counted). (E) Sfh5 and Snx4 colocalize at endosomal compartments. sec22-2 cells were cotransformed with pUG36GFP-Sfh5 and either pAD54-RFP-Snx4, pSM1960-Sec63-RFP, or pAD54-RFP-Yif1. Cells were grown in medium lacking methionine (1 h) and either maintained at 26°C or shifted for 1 h to 37°C, before visualization. (F) Subcellular fractionation of Sfh5. WT and sec22-2 cells overexpressing GST-Sfh5 (BG1805Sfh5) were grown to mid-log phase, maintained at 26°C or shifted for 1 h to 37°C, and lysed. Lysates were subjected to differential centrifugation to obtain P10, S10, P100, and S100 fractions (see Materials and Methods). Samples of the fractions and TCLs were resolved by SDS-PAGE and detected in blots using anti-GST, -Dpm1, -Sso1, -Sed5, and -Ufe1 antibodies. (G) Quantification of the subcellular distribution of Sfh5. Densitometry was used to quantify the relative amounts of Sfh5 and detected markers in the P100 fraction, after normalization for protein expression. Measurements are expressed in arbitrary units. A representative experiment out of three experiments having similar results is shown.
Figure 7.
PI binding is essential for Sfh5 to transduce the exocytic signal to Cdc42 via Mss4. (A) Alignment of the C-terminals of Sec14 and Sfh2-5. Sec14 and Sfh2-5 were aligned using the ClustW algorithm. Asterisks (*) indicate conserved residues, whereas colons (:) and periods (.) indicate strongly and loosely homologous residues, respectively. Numbers indicate amino acid residues. (B) SFH5 E204A,K236A overexpression fails to rescue the mss4-102 mutant. mss4-102 cells were transformed with control vector, pUG36GFP-Sfh5, pUG36GFP-Sfh5K236A, or pUG36GFP-Sfh5E204A,K236A. Cells were grown overnight in medium lacking methionine, diluted serially, plated, and incubated for 48 h at the indicated temperatures (Celsius). (C) SFH5 E204A,K236A overexpression results in mislocalization of RFP-Cdc42 in WT and sec8-9 cells. WT and sec8-9 cells were cotransformed with pAD54-RFP-Cdc42 and either control vector, pUG36GFP-Sfh5 (SFH5), or pUG36GFP-Sfh5E204A,K236A (SFH5 E204A,K236A). Cells were grown in medium lacking methionine (1 h) and maintained at 26°C or shifted for 1 h to 37°C before visualization. The white tracings indicate the outline of the cells. (D) GFP-Sfh5E204A,K236A is mislocalized in WT and sec8-9 cells. WT and sec8-9 strains were transformed with pUG36GFP-Sfh5E204A,K236A. Cells were grown in medium lacking methionine (1 h) and either maintained at 26°C or shifted for 1 h to 37°C, before visualization. Numbers indicate the percentage of cells in which PM localization was observed (n = 100 cells counted). (E) Expression levels of Sfh5 and Sfh5E204A,K236A. WT and sec8-9 cells transformed either with plasmid pUG36GFP-Sfh5 (Sfh5) or pUG36GFP-Sfh5E204A,K236A (Sfh5EAKA) were grown to mid-log phase in medium lacking methionine. Cells (20 OD600 units) were collected and lysed. Equal samples of 20 μg of the TCLs were resolved by SDS-PAGE, and Sfh5 was detected in the blot using anti-GFP (1:1000) antibodies.
Figure 8.
The exocytic signal conferred by PI signaling and regulation of the actin cytoskeleton. Sfh5, a nonclassical PITP, delivers PI to the PM via the (actin- and myosin-dependent) secretory pathway (1). Upon SNARE-mediated vesicle fusion at the PM (2), Sfh5 delivers PI to the PI 4-kinase and PI(4)P 5-kinase (e.g., Stt4 and Mss4) to generate PI(4,5)P2 (3). PI(4,5)P2 in turn regulates three activation paths that converge on the actin cytoskeleton and secretory pathway (4–6). One path consists of a Cdc42-dependent signaling pathway (4) that regulates polarity establishment and actin organization to perpetuate exocytosis until budding is completed. A second path involves the recruitment of the GEF for Rho proteins, Rom2, by PI(4,5)P2, resulting in activation of the Rho1 signaling pathway (5). Rho1 then activates Pkc1 to regulate a mitogen-activated protein kinase cascade that leads to the up-regulation of genes involved in cell wall integrity, as well as actin organization. Finally, recent studies suggest that certain exocyst components (e.g., Sec3) interact with Rho family GTPases (e.g., Rho3) and are recruited to membranes in a PI(4,5)P2-dependent manner (6). This may control exocyst assembly at the site of exocytosis and facilitate secretion, among other possible functions (i.e., cortical ER delivery and anchoring). Alone, PI(4,5)P2-mediated Cdc42 and Rho activation should stabilize the polarized actin cytoskeleton and, in turn, maintain both integrity of secretory pathway and facilitate further Sfh5-mediated PI delivery to the cell surface.
Similar articles
- The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2.
Fadri M, Daquinag A, Wang S, Xue T, Kunz J. Fadri M, et al. Mol Biol Cell. 2005 Apr;16(4):1883-900. doi: 10.1091/mbc.e04-07-0564. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689497 Free PMC article. - MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae.
Desrivières S, Cooke FT, Parker PJ, Hall MN. Desrivières S, et al. J Biol Chem. 1998 Jun 19;273(25):15787-93. doi: 10.1074/jbc.273.25.15787. J Biol Chem. 1998. PMID: 9624178 - Calmodulin controls organization of the actin cytoskeleton via regulation of phosphatidylinositol (4,5)-bisphosphate synthesis in Saccharomyces cerevisiae.
Desrivières S, Cooke FT, Morales-Johansson H, Parker PJ, Hall MN. Desrivières S, et al. Biochem J. 2002 Sep 15;366(Pt 3):945-51. doi: 10.1042/BJ20020429. Biochem J. 2002. PMID: 12079494 Free PMC article. - Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton.
Tapon N, Hall A. Tapon N, et al. Curr Opin Cell Biol. 1997 Feb;9(1):86-92. doi: 10.1016/s0955-0674(97)80156-1. Curr Opin Cell Biol. 1997. PMID: 9013670 Review. - Inositol lipids as spatial regulators of membrane traffic.
Cockcroft S, De Matteis MA. Cockcroft S, et al. J Membr Biol. 2001 Apr 1;180(3):187-94. doi: 10.1007/s002320010069. J Membr Biol. 2001. PMID: 11337890 Review. No abstract available.
Cited by
- Regulation of yeast polarized exocytosis by phosphoinositide lipids.
Volpiana MW, Nenadic A, Beh CT. Volpiana MW, et al. Cell Mol Life Sci. 2024 Nov 19;81(1):457. doi: 10.1007/s00018-024-05483-x. Cell Mol Life Sci. 2024. PMID: 39560727 Free PMC article. Review. - Avidity-driven polarity establishment via multivalent lipid-GTPase module interactions.
Meca J, Massoni-Laporte A, Martinez D, Sartorel E, Loquet A, Habenstein B, McCusker D. Meca J, et al. EMBO J. 2019 Feb 1;38(3):e99652. doi: 10.15252/embj.201899652. Epub 2018 Dec 17. EMBO J. 2019. PMID: 30559330 Free PMC article. - Non-Vesicular Lipid Transport Machinery in Leishmania donovani: Functional Implications in Host-Parasite Interaction.
Das K, Nozaki T. Das K, et al. Int J Mol Sci. 2023 Jun 26;24(13):10637. doi: 10.3390/ijms241310637. Int J Mol Sci. 2023. PMID: 37445815 Free PMC article. Review. - Role of SEC14-like phosphatidylinositol transfer proteins in membrane identity and dynamics.
Montag K, Ivanov R, Bauer P. Montag K, et al. Front Plant Sci. 2023 May 15;14:1181031. doi: 10.3389/fpls.2023.1181031. eCollection 2023. Front Plant Sci. 2023. PMID: 37255567 Free PMC article. Review. - Lipid Exchangers: Cellular Functions and Mechanistic Links With Phosphoinositide Metabolism.
Lipp NF, Ikhlef S, Milanini J, Drin G. Lipp NF, et al. Front Cell Dev Biol. 2020 Jul 21;8:663. doi: 10.3389/fcell.2020.00663. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793602 Free PMC article. Review.
References
- Aronov S., Gerst J. E. Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J. Biol. Chem. 2004;279:36962–36971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous