Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP - PubMed (original) (raw)
Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP
Gregor Kohlmaier et al. Curr Biol. 2009.
Abstract
The centrosome is the principal microtubule organizing center (MTOC) of animal cells. Accurate centrosome duplication is fundamental for genome integrity and entails the formation of one procentriole next to each existing centriole, once per cell cycle. The procentriole then elongates to eventually reach the same size as the centriole. The mechanisms that govern elongation of the centriolar cylinder and their potential relevance for cell division are not known. Here, we show that the SAS-4-related protein CPAP is required for centrosome duplication in cycling human cells. Furthermore, we demonstrate that CPAP overexpression results in the formation of abnormally long centrioles. This also promotes formation of more than one procentriole in the vicinity of such overly long centrioles, eventually resulting in the presence of supernumerary MTOCs. This in turn leads to multipolar spindle assembly and cytokinesis defects. Overall, our findings suggest that centriole length must be carefully regulated to restrict procentriole number and thus ensure accurate cell division.
Figures
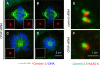
Figure 1. CPAP is required for centriole duplication in cycling cells
(A-D) U2-OS cells transfected with CPAP siRNAs for 72 h and stained for α-tubulin (green), Centrin-3 (red). In this and other figure panels, DNA is viewed in blue and insets show higher magnification views of denoted regions of interest. Two representative bipolar (A-B) and monopolar (C-D) figures are shown. (E-F) U2-OS cells treated with the indicated siRNAs for 72 h, a synchronized by a thymidine block for the last 24 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). 73% of cells treated with control siRNAs displayed HsSAS-6 signals near Centrin-foci (N=226), compared to 76% of cells treated with CPAP siRNA with a single Centrin focus (N=54).
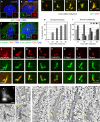
Figure 2. CPAP overexpression induces the formation of abnormal elongated centrioles
(A-B) U2-OS cells not transfected (A) or transiently transfected with GFP-CPAP for 72 h (B) and stained with antibodies against GFP (green) and CPAP (red). (C-I) i-GFP-CPAP cells induced with doxycycline for 0, 24, 48, 72 or 96 h prior to fixation and stained with antibodies against GFP (green) and CPAP (red). Representative thread configurations are shown in (C-G). Panel (H) shows the frequency of threads (N>250 at each time point), panel (I) their complexity, as determined by the number of free ends per thread. (J-K) U2-OS cells transiently transfected with GFP-CPAP for 72 h, incubated for 1 h at 4°C and stained with antibodies against α-tubulin (green) and GFP (red) (J), or with antibodies against acetylated tubulin (green) and GFP (red). (L-S) Representative high magnification images of threads from i-GFP-CPAP cells at different stages of the cell cycle induced with doxycycline for 48 h and stained with antibodies against GFP (green) and polyglutamylated (pg.) tubulin (L), Cep135 (M), Centrin-3 (N), CP110 (O), HsSAS-6 (P), C-Nap1 (Q), Odf2 (R) or Ninein (S), respectively (each in red). (T) i-GFP-CPAP cell 72 hours after induction with doxycycline. Maximal-intensity projection of a through-focus series. Size bar is 500 nm. (U-V) Two sequential 100-nm thin EM sections through the central part of the centrosome in the same cell. Both mother and daughter centrioles are elongated and their distal ends are distorted (asterisks in V mark distal ends of individual microtubule blades). Two procentrioles (arrows) associate with the mother centriole, one near the proximal end, one near the distal end. There is also an electron-dense thread located ∼600 nm away from the distal end of the mother centriole (arrowhead in V), which corresponds to a lower-intensity CPAP-GFP signal (arrowhead in T). (W) Individual 2.6-nm slices from the tomogram of the section presented in (V). Tomography reveals that the electron-dense thread denoted with an arrowhead in (V) corresponds to two parallel microtubule doublets (arrowheads in 1) not connected to the centriole. Both procentrioles are poorly organized and contain only individual microtubules (arrows in 2 and 3). (X-Z’) 15-nm tomography slices illustrating the structure of an elongated mother centriole (see Fig. S4 for complete series of 100-nm thin sections; see also Movie S1). The proximal part of the centriole is cylindrical (Y-Z’), while the distal part is severely distorted. The blades are formed by microtubule doublets and some of the blades are discontinuous (arrow in Y). The entire length of this centriole is embedded in prominent electron-opaque pericentriolar material (PCM). Several sub-distal appendages are present on one side of the centriole (arrowheads in Y-Z’, see also Fig. S4E). In addition to the regular procentriole (see Fig. S4I-J), an ectopic procentriole is present near the distal end of this centriole (arrow in Z). Size bar is 250 nm.
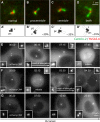
Figure 3. Elongation of the procentriole or the centriole occurs in G2 or shortly thereafter
(A-D) i-GFP-CPAP cells induced with doxycycline for 18 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). Centrioles (c) and procentrioles (pc) are drawn schematically, and overly long centriolar cylinders are indicated in white (A’-D’). Percentages represent fraction of each sort among centrosomes with threads. In 16/50 thread-bearing centrosomes, the single thread could not be unambiguously assigned to either procentriole or centriole, resulting in a slight underestimate of the frequency of single elongation events. Note also that 6% of uninduced prophase cells exhibited threads, all stemming from the centriole. (E-F) Live imaging of cycling i-mCherry-CPAP cells. In this and other live imaging panels, time is denoted in hours:minutes, with 00:00 corresponding to the onset of the time-lapse recording. Threads are first detected at 06:15 in (E), before mitosis, and at 03:30 in (F), after mitosis. See also Movies S2 and S3. (G) Live imaging of i-mCherry-CPAP cell held in 2 mM hydroxyurea (HU) for 24 hours prior to incubation with both HU and doxycycline for 24 hours and onset of filming. Note that threads do not form during the entire duration of the recording, which also indicates that thread formation in cycling cells is not simply due to continued exposure to elevated CPAP levels. See also Movie S4. This results was observed in 4/5 cells held in HU, whereas 1/5 cell formed threads as CPAP levels became extremely elevated before cell death. Related results were obtained by immunofluorescence analysis of cells held in S phase: 22% of cycling i-GFP-CPAP U2-OS cells (N=154) harbored threads 48 hours after induction by doxycyclin, compared to 3% (N=125) when held in thymidine.
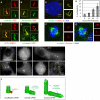
Figure 4. CPAP overexpression results in excess PCM recruitment, supernumerary procentrioles and defective cell division
(A-C) Representative high magnification images from i-GFP-CPAP cells induced with doxycycline for 48 h and stained with antibodies against GFP (red) and γ-tubulin (A), pericentrin (B) or NEDD1 (C) (each in green). (D) U2-OS cell transiently transfected with GFP-CPAP for 72 h, subjected to cold-induced depolymerization for 1 h and warmed up to 37°C for 4 min prior to staining with antibodies against α-tubulin (green) and GFP (red). Arrows point to microtubules emanating from threads. (E) i-GFP-CPAP cells induced with doxycycline for 72 h and stained with antibodies against Centrin-2 (green) and HsSAS-6 (red). Arrows point to procentrioles (1 and 2 correspond to regular procentrioles and 3 to an ectopic procentriole formed in the vicinity of the overly long centriole). (F-G) i-GFP-CPAP cells induced with doxycycline for 72 h and stained with antibodies against α-tubulin (green), Centrin-3 (red). Note that some cells with threads assemble a bipolar spindle (see Fig. S3B), and that some cells that do not harbor a thread assembled a multipolar spindle (data not shown). (H) Fraction of multipolar spindles among i-GFP-CPAP cells induced with doxycycline for 0, 24, 48, or 72 h prior to fixation and staining with antibodies against α-tubulin. Data from two independent experiments were pooled (n>100 for each). (I) Selected fluorescence (maximal-intensity projections) frames from time-lapse recordings of i-mCherry-CPAP cell. Note bipolar spindle assembly at 03:45, presence of multiple individual threads in the ensuing G1 (06:00) and multipolar spindle assembly at the next cell division (22:15). See also Movie S5, where corresponding DIC frames are shown in addition. (J) CPAP is critical for centriole elongation. Whereas CPAP depletion (left) results in a procentriole that harbors HsSAS-6 but not more distal centriolar proteins, and presumably no microtubules, excess CPAP results in overly long centriolar cylinders (right). Note that HsSAS-6 is present in all three cases, but is less visible inside the centriole cylinder in the two rightmost panels.
Similar articles
- PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle.
Chang J, Cizmecioglu O, Hoffmann I, Rhee K. Chang J, et al. EMBO J. 2010 Jul 21;29(14):2395-406. doi: 10.1038/emboj.2010.118. Epub 2010 Jun 8. EMBO J. 2010. PMID: 20531387 Free PMC article. - CPAP is a cell-cycle regulated protein that controls centriole length.
Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK. Tang CJ, et al. Nat Cell Biol. 2009 Jul;11(7):825-31. doi: 10.1038/ncb1889. Epub 2009 Jun 7. Nat Cell Biol. 2009. PMID: 19503075 - The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation.
Tang CJ, Lin SY, Hsu WB, Lin YN, Wu CT, Lin YC, Chang CW, Wu KS, Tang TK. Tang CJ, et al. EMBO J. 2011 Oct 21;30(23):4790-804. doi: 10.1038/emboj.2011.378. EMBO J. 2011. PMID: 22020124 Free PMC article. - Mechanisms of procentriole formation.
Strnad P, Gönczy P. Strnad P, et al. Trends Cell Biol. 2008 Aug;18(8):389-96. doi: 10.1016/j.tcb.2008.06.004. Epub 2008 Jul 10. Trends Cell Biol. 2008. PMID: 18620859 Review. - Cell division: The renaissance of the centriole.
Marshall WF, Rosenbaum JL. Marshall WF, et al. Curr Biol. 1999 Mar 25;9(6):R218-20. doi: 10.1016/s0960-9822(99)80133-x. Curr Biol. 1999. PMID: 10209087 Review.
Cited by
- PPP1R35 is a novel centrosomal protein that regulates centriole length in concert with the microcephaly protein RTTN.
Sydor AM, Coyaud E, Rovelli C, Laurent E, Liu H, Raught B, Mennella V. Sydor AM, et al. Elife. 2018 Aug 31;7:e37846. doi: 10.7554/eLife.37846. Elife. 2018. PMID: 30168418 Free PMC article. - Modeling Human Primary Microcephaly With hiPSC-Derived Brain Organoids Carrying CPAP-E1235V Disease-Associated Mutant Protein.
An HL, Kuo HC, Tang TK. An HL, et al. Front Cell Dev Biol. 2022 Mar 2;10:830432. doi: 10.3389/fcell.2022.830432. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35309908 Free PMC article. - Centrosome Loss Triggers a Transcriptional Program To Counter Apoptosis-Induced Oxidative Stress.
Poulton JS, McKay DJ, Peifer M. Poulton JS, et al. Genetics. 2019 May;212(1):187-211. doi: 10.1534/genetics.119.302051. Epub 2019 Mar 13. Genetics. 2019. PMID: 30867197 Free PMC article. - Epigenetic Signatures of Centrosomes Are Novel Targets in Cancer Diagnosis: Insights from an Analysis of the Cancer Genome Atlas.
Zhang Z, Zhang W. Zhang Z, et al. Epigenomes. 2022 Jun 2;6(2):14. doi: 10.3390/epigenomes6020014. Epigenomes. 2022. PMID: 35735471 Free PMC article. - The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6.
Persico V, Migliorini M, Callaini G, Riparbelli MG. Persico V, et al. Cells. 2020 Jan 3;9(1):115. doi: 10.3390/cells9010115. Cells. 2020. PMID: 31947732 Free PMC article.
References
- Azimzadeh J, Bornens M. Structure and duplication of the centrosome. Journal of Cell Science. 2007;120:2139–2142. - PubMed
- Kirkham M, Müller-Reichert T, Oegema K, Grill S, Hyman A. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112:575–587. - PubMed
- Strnad P, Gönczy P. Mechanisms of procentriole formation. Trends in Cell Biology. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM059363/GM/NIGMS NIH HHS/United States
- BB/D524475/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D012201/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM066270/GM/NIGMS NIH HHS/United States
- GM59363/GM/NIGMS NIH HHS/United States
- GM06627/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources