Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response - PubMed (original) (raw)
Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response
Bernard Charroux et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Drosophila hemocytes have strong phagocytic capacities and produce antimicrobial peptides (AMPs). However, the precise role of blood cells during immune responses and developmental processes has only been studied using indirect means. To overcome this limitation, we generated plasmatocyte-depleted flies by specifically overexpressing the proapoptotic protein Hid into plasmatocytes. Unexpectedly, these plasmatocyte-depleted animals have a normal larval and pupal development and do not exhibit any obvious defect after birth. Remarkably, plasmatocyte-depleted adults show a strong susceptibility to infections by various microorganisms, although activation of systemic AMP gene transcription via the Toll and immune deficiency (IMD) pathways is wild-type. Our data show that this susceptibility, which correlates with overproliferation of bacteria, is likely due to the absence of phagocytosis. We also demonstrate that during larval stages, plasmatocytes play an essential role in mediating AMP production by the fat body after oral bacterial infection. Finally, we show that plasmatocytes are involved in immune surveillance during pupal development, because they prevent bacterial infection that causes pupal lethality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
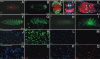
Obtainment of plasmatocyte-depleted Drosophila. Although numerous Croquemort-positive cells are present in hml(Δ)-Gal4, UAS-EGFP embryos (A), these cells do not express GFP. Gal4 expression, visualized through GFP signal, is present in hml(Δ)-Gal4, UAS-EGFP first- (B) and third-instar larvae (F) and in adults (G). A magnification of a hml(Δ)-Gal4, UAS-EGFP/dome-MESO–LacZ (labeling the medullary zone) third-instar larval lymph gland (C) shows that only peripheral differentiated cells from the cortical zone express Gal4. (D and E) Two populations of GFP-positive cells are found in the larval gut. One is located in the proventriculus and surrounds the digestive tract (dashed lines) (D). The second is found in the most posterior region of the ventriculus (E). Some blood cells (arrow) span the entire gut epithelium and therefore contact both the gut lumen and the hemolymph. Ectopic expression of the proapoptotic protein Hid using the hml(Δ)-Gal4 driver gives rise to larvae (H) and adults (I) that are completely devoid of GFP-positive plasmatocytes. (J–M) When bled onto a coverslip, hemolymph from hml(Δ)-Gal4, UAS-EGFP, UAS-hid/+ larvae (L) contain approximately 40% of the number of DAPI-positive cells observed in hml(Δ)-Gal4, UAS-EGFP/+ control larvae (J). These cells are GFP negative (compare K with M), suggesting that they are dying. This is confirmed by the fact that more than 96% of these DAPI-positive cells are TUNEL positive (P and Q). This number is less than 5% in hml(Δ)-Gal4, UAS-EGFP/+ control larvae (N and O).
Fig. 2.
Plasmatocyte-depleted adults are susceptible to various microorganisms. Survival curves after infection by pricking with various microorganisms. E shows that spätzle mutant flies simultaneously plasmatocyte-depleted have a stronger susceptibility to infection by E. faecalis than spätzle mutants. Error bars are shown as vertical bars. Survival curves with an asterisk (*) are statistically significantly different (P < 0.05) from controls.
Fig. 3.
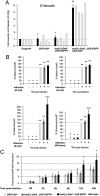
AMP production in plasmatocyte-depleted flies. (A) Bacterial growth after infection by E. faecalis, presented as the relative number of E. faecalis present per fly 20 h after infection. Gray bars, independent experiments; black bars, mean + SD. *P > 0.05. Other differences are statistically significant (**P < 0.05). Similar results were obtained with other Gram-positive bacteria, such as _S. aureus_. (_B_) _AMP_ mRNA inductions are shown in control and plasmatocyte-depleted adults. mRNAs inducibility measured in control flies (_UAS-hid_/+) was set to 100, and values obtained with other genotypes are expressed as a percentage of this value. Each histogram corresponds to the mean value of 6 experiments in which the SD is shown. Values indicated by identical symbols (*, ***, or ***) are not significantly different (_P_ > 0.05) from one another. All other differences are statistically significant (P < 0.05). Similar results were obtained with other Gram-positive bacteria, such as _S. aureus_ and _E. faecalis_ (not shown). (_C_) Quantitative RT-PCR analysis of _Drosomycin_ induction after _M. luteus_ septic injury in adults. Relative ΔCt_Drosomycin_/ΔCt_rp49_ ratios of unchallenged _UAS-hid_/+ controls were anchored in 1 to indicate fold induction. Graphs represent the mean and SD of relative ratios detected in 3 biologic repetitions of a pool of 5 females. Histograms with a single asterisk (*) are significantly (_P_ < 0.05) different from control genotypes. All other differences are not statistically significant (_P_ > 0.05).
Fig. 4.
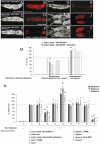
Larval plasmatocytes are required for full induction of an immune systemic response after oral infection. Larvae were orally infected with E. carotovora and analyzed for Dpt-cherry expression. Three classes of larvae were observed: (i) larvae showing IMD pathway activation in the entire fat body (A and B), (ii) larvae in which the reporter is only expressed in the anterior and posterior fat body lobes (E and F), and (iii) larvae in which no reporter activation is noticeable (data not shown). When plasmatocyte-depleted larvae were orally infected, the number of nonresponsive larvae increases at the expense of larvae with global fat body IMD pathway activation (M). Each histogram corresponds to the mean value of 5 independent experiments (200 larvae analyzed per experiment) in which the SD is shown. Empty bars, independent experiments; dark gray bars, mean ± SD. Histograms with a single asterisk (*) are not significantly different (P > 0.05) from controls. Histograms with two asterisks (**) are statistically significant (P < 0.05) from controls. Whereas fat body of control or plasmatocyte-depleted larvae exhibited a uniform expression of _Dpt-cherry_ in the fat body lobes (_K_ and _L_), this expression was patchy in _domino_ fat bodies with fewer cells expressing the transgene (_G–J_). (_N_) _AMP_ mRNA inductions are shown in control, plasmatocyte-depleted, _eater_, _l (3)hem_, and _domino_ mutant larvae orally infected with _E. carotovora. AMP_ mRNA inducibility measured in control flies (_UAS-hid_/+) was set to 100, and values obtained with other genotypes are expressed as a percentage of this value. Histograms correspond to the mean ± SD of 5 experiments. *_P_ > 0.05. Other differences are statistically significant (**P < 0.05).
Fig. 5.
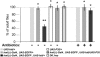
Lethality of plasmatocyte-depleted pupae can be rescued by antibiotics treatment. More than 50% of the hml(Δ)-Gal4, UAS-GFP, UAS-hid/+ individuals die during postembryonic development, during the pupal stage. This lethality is suppressed by coexpression of the antiapoptotic protein P35, demonstrating the specificity of the effect observed. When plasmatocyte-depleted flies are raised on food supplemented by antibiotics, most of the pupal lethality is suppressed. The number of adult flies of the control genotype UAS-hid/+ was set up to 100, and values obtained with other genotypes are expressed as a percentage of this value. Histograms correspond to the mean ± SD of 5 experiments. *P > 0.05. Other differences are statistically significant (**P < 0.05).
Similar articles
- Apoptosis in Hemocytes Induces a Shift in Effector Mechanisms in the Drosophila Immune System and Leads to a Pro-Inflammatory State.
Arefin B, Kucerova L, Krautz R, Kranenburg H, Parvin F, Theopold U. Arefin B, et al. PLoS One. 2015 Aug 31;10(8):e0136593. doi: 10.1371/journal.pone.0136593. eCollection 2015. PLoS One. 2015. PMID: 26322507 Free PMC article. - Drosophila metamorphosis involves hemocyte mediated macroendocytosis and efferocytosis.
Ghosh S, Ghosh S, Mandal L. Ghosh S, et al. Int J Dev Biol. 2020;64(4-5-6):319-329. doi: 10.1387/ijdb.190215lm. Int J Dev Biol. 2020. PMID: 32658992 Free PMC article. - Drosophila immune cell migration and adhesion during embryonic development and larval immune responses.
Ratheesh A, Belyaeva V, Siekhaus DE. Ratheesh A, et al. Curr Opin Cell Biol. 2015 Oct;36:71-9. doi: 10.1016/j.ceb.2015.07.003. Epub 2015 Jul 24. Curr Opin Cell Biol. 2015. PMID: 26210104 Review. - Subpopulation of Macrophage-Like Plasmatocytes Attenuates Systemic Growth via JAK/STAT in the Drosophila Fat Body.
Shin M, Cha N, Koranteng F, Cho B, Shim J. Shin M, et al. Front Immunol. 2020 Jan 31;11:63. doi: 10.3389/fimmu.2020.00063. eCollection 2020. Front Immunol. 2020. PMID: 32082322 Free PMC article. - There and back again: The mechanisms of differentiation and transdifferentiation in Drosophila blood cells.
Csordás G, Gábor E, Honti V. Csordás G, et al. Dev Biol. 2021 Jan 1;469:135-143. doi: 10.1016/j.ydbio.2020.10.006. Epub 2020 Oct 23. Dev Biol. 2021. PMID: 33131706 Review.
Cited by
- Immune Control of Animal Growth in Homeostasis and Nutritional Stress in Drosophila.
P P, Tomar A, Madhwal S, Mukherjee T. P P, et al. Front Immunol. 2020 Jul 31;11:1528. doi: 10.3389/fimmu.2020.01528. eCollection 2020. Front Immunol. 2020. PMID: 32849518 Free PMC article. - An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis.
Avet-Rochex A, Boyer K, Polesello C, Gobert V, Osman D, Roch F, Augé B, Zanet J, Haenlin M, Waltzer L. Avet-Rochex A, et al. BMC Dev Biol. 2010 Jun 11;10:65. doi: 10.1186/1471-213X-10-65. BMC Dev Biol. 2010. PMID: 20540764 Free PMC article. - Gut-derived peptidoglycan remotely inhibits bacteria dependent activation of SREBP by Drosophila adipocytes.
Charroux B, Royet J. Charroux B, et al. PLoS Genet. 2022 Mar 4;18(3):e1010098. doi: 10.1371/journal.pgen.1010098. eCollection 2022 Mar. PLoS Genet. 2022. PMID: 35245295 Free PMC article. - Pseudomonas aeruginosa RhlR is required to neutralize the cellular immune response in a Drosophila melanogaster oral infection model.
Limmer S, Haller S, Drenkard E, Lee J, Yu S, Kocks C, Ausubel FM, Ferrandon D. Limmer S, et al. Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17378-83. doi: 10.1073/pnas.1114907108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987808 Free PMC article. - Phagocytic ability declines with age in adult Drosophila hemocytes.
Horn L, Leips J, Starz-Gaiano M. Horn L, et al. Aging Cell. 2014 Aug;13(4):719-28. doi: 10.1111/acel.12227. Epub 2014 May 14. Aging Cell. 2014. PMID: 24828474 Free PMC article.
References
- Evans CJ, Banerjee U. Transcriptional regulation of hematopoiesis in Drosophila. Blood Cells Mol Dis. 2003;30:223–228. - PubMed
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: Conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. - PubMed
- Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. - PubMed
- Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. - PubMed
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases