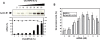Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth - PubMed (original) (raw)
Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth
Laurence Crombez et al. Nucleic Acids Res. 2009 Aug.
Abstract
The development of short interfering RNA (siRNA), has provided great hope for therapeutic targeting of specific genes responsible for pathological disorders. However, the poor cellular uptake and bioavailability of siRNA remain a major obstacle to their clinical development and most strategies that propose to improve siRNA delivery remain limited for in vivo applications. In this study, we report a novel peptide-based approach, MPG-8 an improved variant of the amphipathic peptide carrier MPG, that forms nanoparticles with siRNA and promotes their efficient delivery into primary cell lines and in vivo upon intra-tumoral injection. Moreover, we show that functionalization of this carrier with cholesterol significantly improves tissue distribution and stability of siRNA in vivo, thereby enhancing the efficiency of this technology for systemic administration following intravenous injection without triggering any non-specific inflammatory response. We have validated the therapeutic potential of this strategy for cancer treatment by targeting cyclin B1 in mouse tumour models, and demonstrate that tumour growth is compromised. The robustness of the biological response achieved through this approach, infers that MPG 8-based technology holds a strong promise for therapeutic administration of siRNA.
Figures
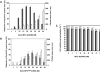
Figure 1.
MPG-8 nanoparticle-mediated delivery of siRNA targeting cyclin B1. Impact of MPG-8 particle size on silencing efficiency (A and B): A fixed concentration of 20 nM of siRNA (Cyc-B1) was associated with different molar ratios of MPG-8 (A) or MPG (B) ranging from 1/1 to 50/1. The size of the MPG-8/siRNA or MPG/siRNA particles were measured by light scattering (white bars) and the biological response associated with siRNA internalization was evaluated in cultured cells by measuring reduction of cyclin B1 protein levels 24 h after transfection (grey bars). Toxicity of MPG-8 particles (C): The toxicity of MPG-8 particles was investigated by MTT assay (grey bars) and by monitoring the level of cyclophilin mRNA (white bars). HeLa cells were treated with increasing concentrations of MPG-8/siRNA particles ranging from 1 to 100 µM and toxicity was then evaluated 12 h (Cyclophilin mRNA) or 24 h (MTT) after treatment. Reported data are the average of three separate experiments.
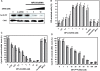
Figure 2.
MPG-8-mediated delivery of siRNA targeting cyclin B1 induces G2-arrest. MPG-8 (A and B) and MPG (C) dose–response of Cyclin B1 silencing at the protein and mRNA levels. Stock solutions of MPG-8/siRNA (100 nM) or MPG/siRNA (500 nM) particles were prepared at a molar ratio of 1/20, and lower concentrations (from 200 nM to 0.125 nM) were obtained by serial dilution of the stock solution in PBS. HeLa (A) and HS-68 (B and C) cells (60% confluency) were overlaid with preformed complexes for 30 min, then fresh DMEM supplemented with 10% FCS was added directly to the cells, which were then returned to the incubator for 24 h. Cyclin B1 protein levels were determined by western blotting using Cdk2 as a control for quantification (grey bars). Cyclin B1 mRNA levels were measured 12 h after transfection using Quantigen technology (white bars). Mismatched Cyc-B3 siRNA associated with MPG-8 (200 nM) and empty MPG-8 particles (20 µM) were used as a control. Dose–response of G2-arrest associated with Cyclin B1 silencing (D). HeLa (grey bars) and HS68 (white bars) cells were treated with increasing concentrations of MPG-8/siRNA-Cyc-B1 from 0.25 to 20 nM. The cell cycle status was evaluated by FACS analysis. Mismatched Cyc-B3 siRNA (100 nM) and GAPDH siRNA (100 nM) associated to MPG-8 as well as to MPG-8 carrier alone (20 µM) were used as controls. Results are the means ± of four separate experiments.
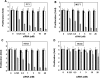
Figure 3.
Cyclin B1 siRNA-MPG-mediated delivery reduces cancer cell proliferation. PC3 (A), MCF7 (B) SKBR3-HER2 (C) tumour cell lines and HS-68 (D) were treated on Day 1, with increasing concentrations of MPG-8/siRNA-Cyc-B1 (from 0.125 to 20 nM). The concentrations of formulated siRNA (from 20 to 0.125 nM) were obtained by serial dilution as described in Figure 2. The proliferation of cells treated with MPG-8/siRNA (grey bars) was evaluated on Day 7 and compared to controls including a MPG-8/Cyc-B3 mismatch siRNA (black bars) and MPG-8 carrier alone (white bars). Results are the average of four independent experiments.
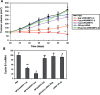
Figure 4.
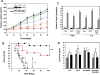
MPG-8-mediated Cyclin B1 siRNA delivery inhibits tumour growth upon intra-tumoural injection. (A) Swiss nude mice (a cohort of N = 6 animals) were injected subcutaneously with 106 PC3 cells. Thirty days after tumour implant, when tumour size reached 100 mm3, animals were treated by intratumoral injection, every 3 days, with a solution of 0.1 ml of either free Cyc-B1 siRNA (100 µg) (in blue), control siRNA Cyc-B3 (50 µg, in green), Cyc-B1 siRNA (1 µg in orange and 5 µg in red) complexed with MPG-8 at a 1/20 molar ratio, or Cyc-B1 siRNA (5 µg, in purple) associated with MPG at a 1/20 molar ratio. Curves show the mean value of tumour size. (B) After 48 days, PC3 tumours were removed, and Cyclin B1 mRNA levels were evaluated by Quantigen and normalized to GAPDH levels. *P < 0.05 versus saline control and **P < 0.01 versus saline control.
Figure 5.
Cholesterol functionalization of MPG-8 does not affect ex-vivo efficiency. (A) Cholesterol functionalization of MPG-8-based particles. Formulations containing variable concentrations of Chol-MPG-8 were obtained by forming a precomplex of MPG-8/siRNA at a molar ratio of 1/20 and then increasing the ratio of siRNA/carrier up to 1/25 with Chol-MPG-8. The impact of the Chol-MPG-8 concentration on particle efficiency was evaluated using 5 nM Cyc-B1 siRNA and increasing concentrations of cholesterol-functionalized MPG-8, by measuring Cyclin B1 protein levels by western blot analysis 24 h after transfection (A, top) and mRNA levels by Quantigen assay (A, bottom). mRNA levels were corrected using cyclophilin level as control (closed circles). (B) Dose–response of G2-arrest associated with Cyclin B1 silencing. HeLa cells were treated with increasing concentrations of MPG-8/siRNA-Cyc-B1 (grey bars) and of MPG-8/siRNA-Cyc-B1/chol-MPG-8 (white bars) as in Figure 2. The concentrations of formulated siRNA (from 20 to 0.25 nM) were obtained by serial dilution of the stock solution in PBS. The cell cycle status was evaluated by FACS analysis. Results are the means ± of four separate experiments.
Figure 6.
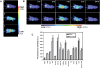
In vivo biodistribution of MPG-8 and cholesterol functionalization of MPG-8. Mice were injected intravenously with 10 µg (200 µl) of Alexa700 fluorescently labelled siRNA either naked (A, 1) or complexed with MPG-8 (A, 2) or MPG-8Chol/MPG-8 (A, 3). Anaesthetized mice were illuminated by 663 nm light emitting diodes equipped with interference filters and fluorescence images and real-time kinetics of biodistribution were acquired during the first 15 min with time of exposures ranging from 100 to 200 ms/image (A), then every hour for 5 h and after 24 h (B). The level of fluorescently labelled siRNA in the different organs was quantified 24 h after administration (C). Results are the average of three animals per group.
Figure 7.
Systemic administration of MPG-8/MPG-8chol/cyclin B1 siRNA blocks tumour growth in vivo. (A) Inhibition of PC3 tumour growth upon intravenous injection. Swiss nude nice (a cohort of N = 6 animals) were injected subcutaneously with 106 PC3 cells and tumour analysis was performed as described in Figure 4A. Animals were treated by intravenous tail vein injection, every 3 days, with a solution of 0.1 ml of either PBS (black), free Cyc-B1 siRNA (100 µg:blue), Cyc-B1 siRNA (10 µg) complexed with MPG-8 (purple), control siRNA Cyc-B3 (100 µg: green) or Cyc-B1 siRNA (5 µg: orange and 10 µg: red) complexed with MPG-8/chol-MPG-8 at a 1/20 molar ratio. Curves show the mean value of tumour size in a group of six animals. After 48 days, PC3 tumours were removed, and Cyclin B1 protein levels were evaluated by western blotting (insert) in control (lane a), 5 µg siRNA (lane b) and 10 µg siRNA (lane c) complexed with MPG-8/chol-MPG-8 at a 1/20 molar ratio. *P < 0.05 versus saline control and **P < 0.01 versus saline control. (B) Inhibition of SK-BR3 HER2 tumour growth upon intravenous injection. Swiss nude mice (a cohort of N = 10 animals) were injected subcutaneously with 106 SK-BR3 HER2 cells. Ten days after tumour implant, when tumour size reached 100 mm3, animals were treated by intravenous tail vein injection, every 3 days from D10 to D30, then every 10 days, with a solution of 0.1 ml of either PBS (green), Cyc-B1 siRNA (10 µg) complexed with MPG-8 (blue) and Cyc-B3 (100 µg: orange) Cyc-B1 siRNA (10 µg: red) complexed with MPG-8/Chol-MPG-8. Control mice treated by intravenous tail vein injection of (10 µg) Cyc-B1 siRNA complexed with MPG-8/Chol-MPG-8 (black) *P < 0.05 versus saline control and **P < 0.01 versus saline control. (C) Expression of IFN response genes: expression of INF-β and IL8 relative to GAPDH was analysed by quantitative RT–PCR. HeLa, MCF7 and SCK3-Her2 cells were treated with MPG-8 carrier alone (white) (20 µM), 20 nM of Cyc-B1 siRNA associated with MPG-8 (black) or MPG-8/Chol-MPG8 (light grey) particles. Poly(I:C) was used as a positive control to induce interferon response (grey). (D) MPG-8 formulation does not induce interferon response in vivo. MPG-8/siRNA, MPG-8/MPG-8-Chol/siRNA (0.5 mg/kg), and MPG-8 carrier were intravenously injected into mice and IL-6 (grey), TNF-α (white) and IFN-γ (black) induction were measured in the serum 6 h after injection by sandwich ELISA and expressed as pg/ml. (P versus saline controls *<0.05, **<0.01) each value is the mean of three separate experiments.
Similar articles
- A non-covalent peptide-based strategy for ex vivo and in vivo oligonucleotide delivery.
Crombez L, Morris MC, Heitz F, Divita G. Crombez L, et al. Methods Mol Biol. 2011;764:59-73. doi: 10.1007/978-1-61779-188-8_4. Methods Mol Biol. 2011. PMID: 21748633 - A non-covalent peptide-based carrier for in vivo delivery of DNA mimics.
Morris MC, Gros E, Aldrian-Herrada G, Choob M, Archdeacon J, Heitz F, Divita G. Morris MC, et al. Nucleic Acids Res. 2007;35(7):e49. doi: 10.1093/nar/gkm053. Epub 2007 Mar 5. Nucleic Acids Res. 2007. PMID: 17341467 Free PMC article. - Targeting cyclin B1 inhibits proliferation and sensitizes breast cancer cells to taxol.
Androic I, Krämer A, Yan R, Rödel F, Gätje R, Kaufmann M, Strebhardt K, Yuan J. Androic I, et al. BMC Cancer. 2008 Dec 29;8:391. doi: 10.1186/1471-2407-8-391. BMC Cancer. 2008. PMID: 19113992 Free PMC article. - A non-covalent peptide-based strategy for siRNA delivery.
Crombez L, Charnet A, Morris MC, Aldrian-Herrada G, Heitz F, Divita G. Crombez L, et al. Biochem Soc Trans. 2007 Feb;35(Pt 1):44-6. doi: 10.1042/BST0350044. Biochem Soc Trans. 2007. PMID: 17233597 Review. - Everything you always wanted to know about CADY-mediated siRNA delivery* (* but afraid to ask).
Konate K, Rydstrom A, Divita G, Deshayes S. Konate K, et al. Curr Pharm Des. 2013;19(16):2869-77. doi: 10.2174/1381612811319160004. Curr Pharm Des. 2013. PMID: 23140452 Review.
Cited by
- Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery.
Margus H, Padari K, Pooga M. Margus H, et al. Mol Ther. 2012 Mar;20(3):525-33. doi: 10.1038/mt.2011.284. Epub 2012 Jan 10. Mol Ther. 2012. PMID: 22233581 Free PMC article. Review. - Delivery strategies and potential targets for siRNA in major cancer types.
Lee SJ, Kim MJ, Kwon IC, Roberts TM. Lee SJ, et al. Adv Drug Deliv Rev. 2016 Sep 1;104:2-15. doi: 10.1016/j.addr.2016.05.010. Epub 2016 May 31. Adv Drug Deliv Rev. 2016. PMID: 27259398 Free PMC article. Review. - Peptide carriers to the rescue: overcoming the barriers to siRNA delivery for cancer treatment.
Cummings JC, Zhang H, Jakymiw A. Cummings JC, et al. Transl Res. 2019 Dec;214:92-104. doi: 10.1016/j.trsl.2019.07.010. Epub 2019 Jul 29. Transl Res. 2019. PMID: 31404520 Free PMC article. Review. - Lipid and Peptide-Oligonucleotide Conjugates for Therapeutic Purposes: From Simple Hybrids to Complex Multifunctional Assemblies.
Fàbrega C, Aviñó A, Navarro N, Jorge AF, Grijalvo S, Eritja R. Fàbrega C, et al. Pharmaceutics. 2023 Jan 18;15(2):320. doi: 10.3390/pharmaceutics15020320. Pharmaceutics. 2023. PMID: 36839642 Free PMC article. Review. - Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells.
Jo J, Hong S, Choi WY, Lee DR. Jo J, et al. Sci Rep. 2014 Mar 14;4:4378. doi: 10.1038/srep04378. Sci Rep. 2014. PMID: 24625570 Free PMC article.
References
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cell. Nature. 2001;411:494–498. - PubMed
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 2004;3:318–329. - PubMed
- Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous