Molecular orchestration of differentiation and function of regulatory T cells - PubMed (original) (raw)
Molecular orchestration of differentiation and function of regulatory T cells
Li-Fan Lu et al. Genes Dev. 2009.
Abstract
During the last decade, a unique mechanism of negative regulation of immune responses and inflammation by a dedicated population of so-called regulatory T cells (Treg) has become a focus of intensive investigation. Through the discovery of transcription factor Foxp3 as a central molecular determinant of differentiation and function of Treg cells, the complex biology of these cells, including maintenance of immunological tolerance to "self" and regulation of immune responses to pathogens, commensals, and tumors, has become amenable to mechanistic studies. In this review, we discuss the molecular aspects of Treg cell lineage commitment, maintenance, and function.
Figures
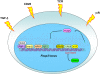
Figure 1.
Regulation of Foxp3 expression. Foxp3 induction is dependent on an increased TCR signal strength combined with stimulation through CD28, and common γ chain cytokine receptors. TGF-β receptor signaling is required for differentiation of peripheral Treg cells, whereas its involvement in Treg differentiation in the thymus is subject of debate. As a consequence, corresponding downstream transcriptional factors are recruited to the Foxp3 locus leading to Foxp3 expression. Foxp3 itself is also suggested to stabilize its own expression. Moreover, complete demethylation of a CpG island within a conserved intronic noncoding Foxp3 element is also required for stable Foxp3 expression, whereas methylation of this CpG island by Dnmt1 in TGF-β-induced Treg cells likely results in a loss of Foxp3.
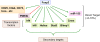
Figure 2.
Foxp3-dependent transcriptional program. Foxp3 acts as both transcriptional activator and repressor. Whereas more genes are up-regulated by Foxp3 (green) in Treg cells, a number of genes are down-regulated (red) in a Foxp3-dependent manner. Genome-wide analysis of Foxp3-binding sites revealed that a relatively small fraction (∼6%–10%) of Foxp3-dependent genes are direct targets of Foxp3. In addition, Foxp3 controls Treg cell transcriptional program indirectly through modulating expression of a set of genes encoding transcription factors and miRNA.
Figure 3.
miRNA-dependent gene regulation preserves Treg suppressor function in inflammatory settings. miRNA depletion due to ablation of Dicer in Treg cells (green) results in impaired homeostasis and attenuated suppressor function in a healthy heterozygous Dicerfl/flFoxp3cre/+ female mice cohabited by Dicer-deficient (green) Dicer-sufficient Treg cells (blue). (Middle panel) Nevertheless, Dicer-deficient Treg cells (green) are still able to suppress self-reactive effector T cells (red), albeit less efficiently than their wild-type counterparts. (Left panel) Consequently, the mice remain healthy and show no sign of inflammation, similar to their wild-type littermates (Dicerfl/+Foxp3cre/cre). (Right panel) In Dicerfl/flFoxp3cre mice, lack of Dicer-sufficient Treg cells results in an inflammation and a complete loss of suppressor capacity of Dicer-deficient Treg cells. This failure of suppressor function in face of inflammation ultimately leads to fatal immune lesions indistinguishable from that in Treg-deficient mice.
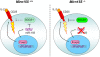
Figure 4.
miR-155-dependent regulation of Treg cell homeostasis. In addition to CD25, Foxp3 induces high level of miR-155 expression to ensure increased IL-2 responsiveness through miR-155-mediated down-regulation of the SOCS1 protein (left panel). In the absence of miR-155 (right panel), increased amounts of the SOCS1 protein attenuate IL-2R signaling, leading to diminished STAT5 phosphorylation and diminished competitive fitness.
Similar articles
- CD4+CD69+ T cells and CD4+CD25+FoxP3+ Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice.
Peixoto TV, Carrasco S, Botte DAC, Catanozi S, Parra ER, Lima TM, Ugriumov N, Soriano FG, de Mello SBV, Rodrigues CM, Goldenstein-Schainberg C. Peixoto TV, et al. Adv Rheumatol. 2019 Jul 24;59(1):30. doi: 10.1186/s42358-019-0072-x. Adv Rheumatol. 2019. PMID: 31340848 - A distal Foxp3 enhancer enables interleukin-2 dependent thymic Treg cell lineage commitment for robust immune tolerance.
Dikiy S, Li J, Bai L, Jiang M, Janke L, Zong X, Hao X, Hoyos B, Wang ZM, Xu B, Fan Y, Rudensky AY, Feng Y. Dikiy S, et al. Immunity. 2021 May 11;54(5):931-946.e11. doi: 10.1016/j.immuni.2021.03.020. Epub 2021 Apr 9. Immunity. 2021. PMID: 33838102 Free PMC article. - Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(-) cells.
Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T. Aarts-Riemens T, et al. Eur J Immunol. 2008 May;38(5):1381-90. doi: 10.1002/eji.200737590. Eur J Immunol. 2008. PMID: 18412171 - Regulatory T cells: mechanisms of differentiation and function.
Josefowicz SZ, Lu LF, Rudensky AY. Josefowicz SZ, et al. Annu Rev Immunol. 2012;30:531-64. doi: 10.1146/annurev.immunol.25.022106.141623. Epub 2012 Jan 6. Annu Rev Immunol. 2012. PMID: 22224781 Free PMC article. Review. - Regulatory T cells: roles of T cell receptor for their development and function.
Ohkura N, Sakaguchi S. Ohkura N, et al. Semin Immunopathol. 2010 Jun;32(2):95-106. doi: 10.1007/s00281-010-0200-5. Epub 2010 Feb 24. Semin Immunopathol. 2010. PMID: 20179931 Review.
Cited by
- Clues to immune tolerance: the monogenic autoimmune syndromes.
Waterfield M, Anderson MS. Waterfield M, et al. Ann N Y Acad Sci. 2010 Dec;1214:138-55. doi: 10.1111/j.1749-6632.2010.05818.x. Epub 2010 Oct 22. Ann N Y Acad Sci. 2010. PMID: 20969580 Free PMC article. Review. - Chronic heat stress inhibits immune responses to H5N1 vaccination through regulating CD4⁺ CD25⁺ Foxp3⁺ Tregs.
Meng D, Hu Y, Xiao C, Wei T, Zou Q, Wang M. Meng D, et al. Biomed Res Int. 2013;2013:160859. doi: 10.1155/2013/160859. Epub 2013 Sep 17. Biomed Res Int. 2013. PMID: 24151582 Free PMC article. - Anti-cancer activity of dose-fractioned mPE +/- bevacizumab regimen is paralleled by immune-modulation in advanced squamous NSLC patients.
Pastina P, Nardone V, Croci S, Battaglia G, Vanni F, Bellan C, Barbarino M, Ricci V, Costantini S, Capone F, Botta C, Zarone MR, Misso G, Boccellino M, Caraglia M, Giordano A, Paladini P, Tassone P, Tagliaferri P, Cusi MG, Pirtoli L, Correale P. Pastina P, et al. J Thorac Dis. 2017 Sep;9(9):3123-3131. doi: 10.21037/jtd.2017.08.68. J Thorac Dis. 2017. PMID: 29221287 Free PMC article. - Histone deacetylase 6 plays an important role in TGF-β-induced murine Treg cell differentiation by regulating cell proliferation.
Lee JH, Kim HS, Jang SW, Lee GR. Lee JH, et al. Sci Rep. 2022 Dec 29;12(1):22550. doi: 10.1038/s41598-022-27230-7. Sci Rep. 2022. PMID: 36581745 Free PMC article. - Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine.
Wang X, Dong L, Ni H, Zhou S, Xu Z, Hoellwarth JS, Chen X, Zhang R, Chen Q, Liu F, Wang J, Su C. Wang X, et al. PLoS Negl Trop Dis. 2013 Apr 4;7(4):e2164. doi: 10.1371/journal.pntd.0002164. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23593527 Free PMC article.
References
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. - PubMed
- Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–1131. - PubMed
- Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol. 2007;37:2378–2389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources