A role for Chk1 in blocking transcriptional elongation of p21 RNA during the S-phase checkpoint - PubMed (original) (raw)
A role for Chk1 in blocking transcriptional elongation of p21 RNA during the S-phase checkpoint
Rachel Beckerman et al. Genes Dev. 2009.
Abstract
We reported previously that when cells are arrested in S phase, a subset of p53 target genes fails to be strongly induced despite the presence of high levels of p53. When DNA replication is inhibited, reduced p21 mRNA accumulation is correlated with a marked reduction in transcription elongation. Here we show that ablation of the protein kinase Chk1 rescues the p21 transcription elongation defect when cells are blocked in S phase, as measured by increases in both p21 mRNA levels and the presence of the elongating form of RNA polymerase II (RNAPII) toward the 3' end of the p21 gene. Recruitment of specific elongation and 3' processing factors (DSIF, CstF-64, and CPSF-100) is also restored. While additional components of the RNAPII transcriptional machinery, such as TFIIB and CDK7, are recruited more extensively to the p21 locus after DNA damage than after replication stress, their recruitment is not enhanced by ablation of Chk1. Significantly, ablating Chk2, a kinase closely related in substrate specificity to Chk1, does not rescue p21 mRNA levels during S-phase arrest. Thus, Chk1 has a direct and selective role in the elongation block to p21 observed during S-phase arrest. These findings demonstrate for the first time a link between the replication checkpoint mediated by ATR/Chk1 and the transcription elongation/3' processing machinery.
Figures
Figure 1.
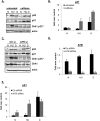
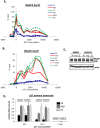
ATR ablation increases p21 protein and mRNA levels when DNA replication is stalled. (A) RKO cells were pretreated or not with 4 mM caffeine for 1 h, and then left untreated (N), treated with HU (HU, 1.7 mM, 24 h) or dauno (D, 0.22 μM, 8 h), lysed, and subjected to immunoblot analysis with indicated antibodies. (B) RKO cells were treated as in A; RNA was extracted and subjected to qRT–PCR using primers for the p21 mRNA transcript. Values were normalized to those of the hprt1 gene. Graphs are representative of at least six independent PCRs from three independent experiments. In all experiments, error bars included in the graphs represent one standard deviation from the mean. (C) RKO cells were transfected with control (Ctr) or ATR siRNA duplexes for 36 h and then drug-treated with HU or dauno as in A. The cells were lysed and subjected to immunoblot analysis with antibodies as indicated in the figure. (D,E) RKO cells were treated as in C, and RNA was extracted and subjected to qRT–PCR as in B using primers for the p21 or ATR mRNA transcript.
Figure 2.
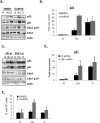
Chk1 ablation rescues p21 protein and mRNA levels during S-phase arrest in HCT116 cells. (A) HCT116 cells were pretreated with DMSO or 1 μM Gö6976 for 1 h, and then left untreated (N), treated with HU (HU, 1.7 mM, 24 h) or dauno (D, 0.22 μM, 8 h), lysed, and subjected to immunoblot analysis with antibodies as indicated in the figure. (B) HCT116 cells were treated as in A; RNA was extracted and subjected to qRT–PCR using primers for the p21. Values were normalized to those of the hprt1 gene. (C) HCT116 cells were transfected with control (Ctr) or Chk1 siRNA duplexes for 36 h and then drug-treated with HU or dauno as in A. Cells were lysed and subjected to immunoblot analysis with antibodies as indicated in the figure. (D) HCT116 cells were treated as in C, RNA was extracted and subjected to qRT–PCR as in B. (E) FACS analysis was performed on HCT116 cells that were subjected to different combinations of drug treatments as in A and the data was analyzed using the ModFit program.
Figure 3.
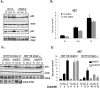
Unlike Chk1, Chk2 exerts a positive effect on p21 transcription. (A) HCT116 cells were transfected with control or Chk2 siRNA duplexes for 36 h, and then left untreated (N), treated with HU (HU, 1.7 mM, 24 h) or dauno (D, 0.22 μM, 8 h), lysed, and subjected to immunoblot analysis with antibodies as indicated in the figure. (B) HCT116 cells were treated as in A, and RNA was extracted and subjected to qRT–PCR using primers for p21. Values are normalized to those of hprt1. (C) HCT116 Chk2+/+ or _Chk2_-null cells were treated as in Figure 1A, lysed, and subjected to immunoblot analysis with the indicated antibodies. Note that the extra band in the dauno-treated _Chk2_-null cells is nonspecific. (D) HCT116 Chk2+/+ or _Chk2_-null cells were treated as in C and RNA was extracted and subjected to qRT–PCR using primers for p21 as in B.
Figure 4.
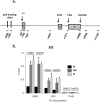
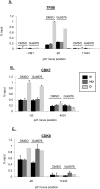
Chk1 inhibition does not affect p53 recruitment to the p21 promoter. (A) Diagram depicting the primers used to amplify the different regions of p21 for ChIP and nascent RNA transcript analyses. (B) ChIP was performed in HCT116 cells following drug treatment as in Figure 2A. In all ChIP experiments, cells were cross-linked in 1% formaldehyde, lysed in RIPA buffer, and sonicated to produce chromatin fragments of ∼500 base pairs. Samples were then immunoprecipitated with anti-p53 monoclonal antibodies (1801 and DO-1). Five percent of the input protein was subjected to immunoblot analysis. ChIP-enriched DNA was quantified by qRT–PCR using the indicated amplicons on p21. Values are expressed as percentage of input DNA immunoprecipitated, normalized to the highest immunoprecipitation signal. For all qChIPs, graphs represent at least six independent PCRs from three separate immunoprecipitations and cell cultures.
Figure 5.
Chk1 inihibition stimulates distal transcription elongation on the p21 locus after HU treatment. (A–C) ChIP was performed in HCT116 cells following treatment as in Figure 2A. Cells were cross-linked in 1% formaldehyde, lysed in RIPA buffer, and sonicated. Lysates were precleared and then immunoprecipitated with antibodies recognizing RNAPII phosphorylated at Ser5 (A) or Ser2 (B) of its heptad repeat, as indicated in the figure. Solid lines refer to samples pretreated with DMSO; dashed lines refer to samples pretreated with Gö6976. Quantification of ChIP-enriched DNA and replicates were performed as in Figure 4. (C) Five percent of the input protein was subjected to immunoblot analysis. (D) HCT116 cells were treated as above; RNA was extracted and subjected to qRT–PCR using primers recognizing different regions of the nascent p21 transcript. Values were normalized to those of hprt1.
Figure 6.
Chk1 inhibition does not affect PIC assembly at the p21 promoter when cells are blocked in S phase. (A–C) ChIP was performed in HCT116 cells following treatment as in Figure 2A. Cells were cross-linked in 1% formaldehyde, lysed in RIPA buffer, and sonicated. Lysates were precleared and then immunoprecipitated with antibodies as indicated in the figure. Five percent of the input protein was subjected to immunoblot analysis. Quantification of ChIP-enriched DNA and replicates were performed as in Figrue 4.
Figure 7.
Chk1 inhibition selectively alters the recruitment of certain factors to the 3′ end of the p21 gene after HU treatment. (A–D) ChIP was performed in HCT116 cells following treatment as in Figure 2A. Cells were cross-linked in 1% formaldehyde, lysed in RIPA buffer, and sonicated. Lysates were precleared and then immunoprecipitated with antibodies as indicated in the figure. Five percent of the input protein was subjected to immunoblot analysis. Quantification of ChIP-enriched DNA and replicates were performed as in Figure 4.
Figure 8.
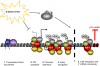
A model for Chk1 function in the transcription elongation block to p21 during S-phase arrest. In cells treated with replication stress, p53 and HAT assembly at the upstream region of the p21 promoter is unaffected. When cells are blocked in S phase, a Chk1-independent differential PIC assembly at p21 is observed, which excludes TFIIB, CDK7, and CDK8 and possibly other components. RNAPII is efficiently recruited to the TATA region of p21 under these conditions, and upon phosphorylation of its Ser5 residue by an unknown kinase (circle with question mark, which is neither CDK7 nor CDK8), it is able to clear the promoter. RNAPII then transits the p21 gene, becoming minimally phosphorylated on Ser2 of its CTD heptad repeats, until it reaches the distal end of the gene (approximately +5 kb to +7 kb), whereupon it encounters a specific, Chk1-regulated, block to elongation. This block correlates with the decreased recruitment of DSIF, CPSF-100, CstF-64 to the distal region on p21. H3K36 trimethylation in this region is also blocked. These conditions lead to greatly reduced p21 mRNA production.
Similar articles
- Chk1 is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21 pathway is functional.
Lossaint G, Besnard E, Fisher D, Piette J, Dulić V. Lossaint G, et al. Oncogene. 2011 Oct 13;30(41):4261-74. doi: 10.1038/onc.2011.135. Epub 2011 May 2. Oncogene. 2011. PMID: 21532626 - Stalled replication induces p53 accumulation through distinct mechanisms from DNA damage checkpoint pathways.
Ho CC, Siu WY, Lau A, Chan WM, Arooz T, Poon RY. Ho CC, et al. Cancer Res. 2006 Feb 15;66(4):2233-41. doi: 10.1158/0008-5472.CAN-05-1790. Cancer Res. 2006. PMID: 16489026 - Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing.
Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, Smythe C. Feijoo C, et al. J Cell Biol. 2001 Sep 3;154(5):913-23. doi: 10.1083/jcb.200104099. J Cell Biol. 2001. PMID: 11535615 Free PMC article. - Evidence that the ATR/Chk1 pathway maintains normal replication fork progression during unperturbed S phase.
Petermann E, Caldecott KW. Petermann E, et al. Cell Cycle. 2006 Oct;5(19):2203-9. doi: 10.4161/cc.5.19.3256. Epub 2006 Oct 1. Cell Cycle. 2006. PMID: 16969104 Review. - The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.
Smith J, Tho LM, Xu N, Gillespie DA. Smith J, et al. Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
Cited by
- CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma.
Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen G, Yang L, Li B, Huang P, Chen D, Liang Y, Zhang R, Pan J, Zeng YX, Kang T. Hong J, et al. J Clin Invest. 2012 Jun;122(6):2165-75. doi: 10.1172/JCI61380. Epub 2012 May 15. J Clin Invest. 2012. PMID: 22585575 Free PMC article. Clinical Trial. - Transcriptional regulation by p53.
Beckerman R, Prives C. Beckerman R, et al. Cold Spring Harb Perspect Biol. 2010 Aug;2(8):a000935. doi: 10.1101/cshperspect.a000935. Epub 2010 Apr 28. Cold Spring Harb Perspect Biol. 2010. PMID: 20679336 Free PMC article. Review. - Long non-coding RNA generated from CDKN1A gene by alternative polyadenylation regulates p21 expression during DNA damage response.
Murphy MR, Ramadei A, Doymaz A, Varriano S, Natelson DM, Yu A, Aktas S, Mazzeo M, Mazzeo M, Zakusilo G, Kleiman FE. Murphy MR, et al. Nucleic Acids Res. 2023 Nov 27;51(21):11911-11926. doi: 10.1093/nar/gkad899. Nucleic Acids Res. 2023. PMID: 37870464 Free PMC article. - CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis.
Zhu Y, Schluttenhoffer CM, Wang P, Fu F, Thimmapuram J, Zhu JK, Lee SY, Yun DJ, Mengiste T. Zhu Y, et al. Plant Cell. 2014 Oct;26(10):4149-70. doi: 10.1105/tpc.114.128611. Epub 2014 Oct 3. Plant Cell. 2014. PMID: 25281690 Free PMC article. - AMP-activated Protein Kinase (AMPK) Control of mTORC1 Is p53- and TSC2-independent in Pemetrexed-treated Carcinoma Cells.
Agarwal S, Bell CM, Rothbart SB, Moran RG. Agarwal S, et al. J Biol Chem. 2015 Nov 13;290(46):27473-86. doi: 10.1074/jbc.M115.665133. Epub 2015 Sep 21. J Biol Chem. 2015. PMID: 26391395 Free PMC article.
References
- Ahn J, Urist M, Prives C. Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem. 2003;278:20480–20489. - PubMed
- Ahn J, Urist M, Prives The Chk2 protein kinase. DNA Repair (Amst) 2004;3:1039–1047. - PubMed
- Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell. 2004;13:67–76. - PubMed
- Bannister AJ, Schneider R, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Spatial distribution of di- and tri-methyl lysine 36 of histone H3 at active genes. J Biol Chem. 2005;280:17732–17736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM028983/GM/NIGMS NIH HHS/United States
- CA77742/CA/NCI NIH HHS/United States
- CA117907/CA/NCI NIH HHS/United States
- P01 CA087497/CA/NCI NIH HHS/United States
- CA87497/CA/NCI NIH HHS/United States
- R01 CA117907/CA/NCI NIH HHS/United States
- R01 CA077742/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous