Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function - PubMed (original) (raw)
Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function
Sui Wang et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Neurog3 (Neurogenin 3 or Ngn3) is both necessary and sufficient to induce endocrine islet cell differentiation from embryonic pancreatic progenitors. Since robust Neurog3 expression has not been detected in hormone-expressing cells, Neurog3 is used as an endocrine progenitor marker and regarded as dispensable for the function of differentiated islet cells. Here we used 3 independent lines of Neurog3 knock-in reporter mice and mRNA/protein-based assays to examine Neurog3 expression in hormone-expressing islet cells. Neurog3 mRNA and protein are detected in hormone-producing cells at both embryonic and adult stages. Significantly, inactivating Neurog3 in insulin-expressing beta cells at embryonic stages or in Pdx1-expressing islet cells in adults impairs endocrine function, a phenotype that is accompanied by reduced expression of several Neurog3 target genes that are essential for islet cell differentiation, maturation, and function. These findings demonstrate that Neurog3 is required not only for initiating endocrine cell differentiation, but also for promoting islet cell maturation and maintaining islet function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
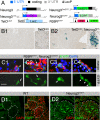
Fig. 1.
Knock-in reporter mice reveal Neurog3 expression in adult islet cells. (A) Knock-in and reporter alleles. TetOLacZ and R26REYFP are reporter alleles of tTA and Cre, respectively. (B) β-Gal expression in 9-week-old TetOLacZ (i) and TetOLacZ;Neurog3tTA (ii) pancreata. (Insets) Islets at a higher magnification. (C) Coexpression of endocrine hormones (red) with EYFP in 8-week-old R26REYFP;Neurog3CreERT males that received TM at 7 weeks of age. Two panels (as a column) for each staining are shown: a merged image of EYFP (green), hormone (red), and DAPI (blue) signal, and a single channel of EYFP. Arrows indicate EYFP+hormone+ cells. (D) EGFP recognized by a rabbit anti-EGFP antibody in 6-month-old Neurog3EGFP pancreas. Only EGFP alone and EGFP-DAPI merged images are shown. (Scale bars, 20 μm.)
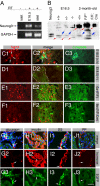
Fig. 2.
Differentiated islet cells express Neurog3. (A) RT-PCR detection of Neurog3 mRNA in 2-month-old WT (+/+) islets and E16.5 embryonic pancreas. GAPDH expression was used as RT control. (B) Western blot detection of Neurog3. Recombinant Neurog3 produced in HEK293 cells, wild-type (+/+), and Neurog3+/− (+/−) E16.5 embryonic pancreata were used as positive controls. E16.5 _Neurog3_−/− (−/−) total pancreas and 2-month-old Neurog3F/F;Ins2Cre (F/F; Cre) islets were used to verify the specificity of the Neurog3 antibodies. All pancreatic samples except the Neurog3F/F lane (F/F, labeled with *) were nuclear extract. Note the presence of the nonspecific bands (red arrows), which serve as internal loading controls of total proteins. The Neurog3 proteins were marked with blue arrows. (C–F) IF detection of Neurog3 in embryonic (E18.5) and 6-week-old pancreata. C-peptide costaining localizes the endocrine compartment. Also note that the Neurog3Hi cells in Cii (arrowheads) do not express insulin c-peptide, but some Neurog3lo cells do (white arrows). Broken lines in Dii show a duct. (G–J) Coexpression of Neurog3EGFP with endocrine hormones in three-month-old pancreas. Note that all 4 major islet cell types express detectable EGFP. White arrows point to examples of EGFP+hormone+ cells. In the “merge” channel, DAPI signal (blue) is included to show the enriched EGFP presence in nuclei. (Scale bars, 20 μm.)
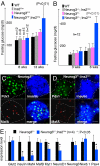
Fig. 3.
β-cell-specific Neurog3 inactivation impairs endocrine function and endocrine gene expression. (A and B) Fasting blood glucose levels in males. The genotypes and ages of animals are labeled. “P” is calculated between the Neurog3F/F and Neurog3F/F;Ins2Cre mice in A and Neurog3 F/− and Neurog3 F/−;Ins2Cre animals in B. (C and D) Insulin, MafA, and Pdx1 expression in E18.5 Neurog3 F/− and Neurog3 F/−;Ins2Cre pancreata. Three single channels and a merged image are presented. Note the percentage of islet cells (Pdx1+) that express MafA (arrows). (Scale bar, 20 μm.) (E) QRT-PCR analysis of the expression of several genes in Neurog3 F/− and Neurog3 F/−;Ins2Cre pancreata (E18.5).
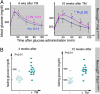
Fig. 4.
Neurog3 inactivation in mature islet cells compromises endocrine function. (A) IPGTT assays after Neurog3 inactivation in Neurog3F/F;Pdx1CreERT female animals. (B) Fasting blood glucose levels in male Neurog3 F/−;Pdx1CreERT animals 4 and 12 weeks after Neurog3 inactivation. Each dot represents 1 animal. Control animals received corn oil vehicle instead of TM.
Fig. 5.
Neurog3 inactivation in mature islet cells compromises expression of insulin, Glut2, and MafA. Gene expression 10 days after TM administration in Neurog3F/F;Pdx1CreERT males is shown. (A) QRT-PCR PCR analysis of gene expression in handpicked islets. (B and C) IF staining of Glut2 and MafA. For each tissue section, a single channel (white) is presented to highlight the relative intensity of the Glut2 (B) or MafA (C) signal, respectively. A merged image is also presented to show the position of the stained islet (recognized by insulin signals). (Scale bar, 20 μm.)
Similar articles
- Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development.
Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G, Gradwohl G. Soyer J, et al. Development. 2010 Jan;137(2):203-12. doi: 10.1242/dev.041673. Development. 2010. PMID: 20040487 Free PMC article. - Ectopic expression of neurogenin 3 in neonatal pig pancreatic precursor cells induces (trans)differentiation to functional alpha cells.
Harb G, Heremans Y, Heimberg H, Korbutt GS. Harb G, et al. Diabetologia. 2006 Aug;49(8):1855-63. doi: 10.1007/s00125-006-0299-z. Epub 2006 May 31. Diabetologia. 2006. PMID: 16736130 - Extensive NEUROG3 occupancy in the human pancreatic endocrine gene regulatory network.
Schreiber V, Mercier R, Jiménez S, Ye T, García-Sánchez E, Klein A, Meunier A, Ghimire S, Birck C, Jost B, de Lichtenberg KH, Honoré C, Serup P, Gradwohl G. Schreiber V, et al. Mol Metab. 2021 Nov;53:101313. doi: 10.1016/j.molmet.2021.101313. Epub 2021 Aug 3. Mol Metab. 2021. PMID: 34352411 Free PMC article. - Revisiting the immunocytochemical detection of Neurogenin 3 expression in mouse and man.
Honoré C, Rescan C, Hald J, McGrath PS, Petersen MB, Hansson M, Klein T, Østergaard S, Wells JM, Madsen OD. Honoré C, et al. Diabetes Obes Metab. 2016 Sep;18 Suppl 1:10-22. doi: 10.1111/dom.12718. Diabetes Obes Metab. 2016. PMID: 27615127 Review. - PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.
Zhu Y, Liu Q, Zhou Z, Ikeda Y. Zhu Y, et al. Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
- Impaired proliferation of pancreatic beta cells, by reduced placental growth factor in pre-eclampsia, as a cause for gestational diabetes mellitus.
Li J, Ying H, Cai G, Guo Q, Chen L. Li J, et al. Cell Prolif. 2015 Apr;48(2):166-74. doi: 10.1111/cpr.12164. Epub 2015 Jan 16. Cell Prolif. 2015. PMID: 25594238 Free PMC article. - PDX1 Methylation, NGN3 and Pax6 Expression Levels in Pregnant Women with Gestational Diabetes Mellitus and their association with neonatal blood sugars and birth weight.
Su Y, Li J, Chen L, Lian H, Qie Y. Su Y, et al. Pak J Med Sci. 2024 Jan-Feb;40(3Part-II):509-513. doi: 10.12669/pjms.40.3.7700. Pak J Med Sci. 2024. PMID: 38356808 Free PMC article. - Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice.
Landsman L, Parent A, Hebrok M. Landsman L, et al. Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17010-5. doi: 10.1073/pnas.1105404108. Epub 2011 Oct 3. Proc Natl Acad Sci U S A. 2011. PMID: 21969560 Free PMC article. - No evidence for β cell neogenesis in murine adult pancreas.
Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, Paredes J, Welsh C, Wiersch J, Gittes GK. Xiao X, et al. J Clin Invest. 2013 May;123(5):2207-17. doi: 10.1172/JCI66323. Epub 2013 Apr 24. J Clin Invest. 2013. PMID: 23619362 Free PMC article. - Directed differentiation of progenitor cells towards an islet-cell phenotype.
Abed A, Critchlow C, Flatt PR, McClenaghan NH, Kelly C. Abed A, et al. Am J Stem Cells. 2012 Nov 30;1(3):196-204. Print 2012. Am J Stem Cells. 2012. PMID: 23671808 Free PMC article.
References
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. - PubMed
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. - PubMed
- Schwitzgebel VM, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 DK072495/DK/NIDDK NIH HHS/United States
- R01 CA106308/CA/NCI NIH HHS/United States
- DK072473/DK/NIDDK NIH HHS/United States
- R01 DK068471/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- R01 DK065949/DK/NIDDK NIH HHS/United States
- R01 DE015654/DE/NIDCR NIH HHS/United States
- DK065949/DK/NIDDK NIH HHS/United States
- DK072495/DK/NIDDK NIH HHS/United States
- DK68471/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases