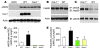Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration - PubMed (original) (raw)
Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration
You-Yang Zhao et al. J Clin Invest. 2009 Jul.
Abstract
Pulmonary hypertension (PH) is an unremitting disease defined by a progressive increase in pulmonary vascular resistance leading to right-sided heart failure. Using mice with genetic deletions of caveolin 1 (Cav1) and eNOS (Nos3), we demonstrate here that chronic eNOS activation secondary to loss of caveolin-1 can lead to PH. Consistent with a role for eNOS in the pathogenesis of PH, the pulmonary vascular remodeling and PH phenotype of Cav1-/- mice were absent in Cav1-/-Nos3-/- mice. Further, treatment of Cav1-/- mice with either MnTMPyP (a superoxide scavenger) or l-NAME (a NOS inhibitor) reversed their pulmonary vascular pathology and PH phenotype. Activation of eNOS in Cav1-/- lungs led to the impairment of PKG activity through tyrosine nitration. Moreover, the PH phenotype in Cav1-/- lungs could be rescued by overexpression of PKG-1. The clinical relevance of the data was indicated by the observation that lung tissue from patients with idiopathic pulmonary arterial hypertension demonstrated increased eNOS activation and PKG nitration and reduced caveolin-1 expression. Together, these data show that loss of caveolin-1 leads to hyperactive eNOS and subsequent tyrosine nitration-dependent impairment of PKG activity, which results in PH. Thus, targeting of PKG nitration represents a potential novel therapeutic strategy for the treatment of PH.
Figures
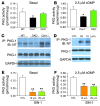
Figure 1. Chronic eNOS activation in Cav1–/– lungs.
(A) Western blot analysis of caveolin-1 and eNOS expression in lungs from 2-month-old mice. Lung lysate (20 μg per lane) was loaded and immunoblotted with antibodies against mouse eNOS, caveolin-1, and β-actin (loading control). Neither caveolin-1 nor eNOS was detected in DKO lungs. (B) Similar protein expression of hsp90 in Cav1–/– and WT lungs. (C) Increased eNOS-hsp90 association in Cav1–/– lungs. Lung lysates (500 μg) were immunoprecipitated with anti-eNOS and immunoblotted with anti-hsp90. The same blot was immunoblotted with anti-eNOS. (D) Quantitative analysis of eNOS-derived NOx in mouse lungs. eNOS-derived NOx was determined using the Griess reagent in the presence of iNOS and nNOS inhibitors but without addition of the eNOS agonist, e.g., calcium ionophore A23187. Data are shown as mean ± SD (n = 4–5). *P < 0.001 versus WT (n = 4–6). Cav1–/– lungs produced 3.5-fold more eNOS-derived NOx than WT lungs. (E) Total NOx production in mouse lungs. Total NOx production was determined with the Griess reagent. Data are expressed as mean ± SD (n = 4–5). †P < 0.05, Cav1–/– versus WT; **P < 0.01, DKO versus WT; #P < 0.05, Nos3–/– versus WT.
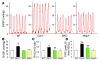
Figure 2. PH and increased PVR in Cav1–/– mice were prevented in DKO mice.
(A) Representative RVSP tracings (each represents 1 second). (B) Normalized RVSP in DKO mice. RVSP was measured in age- and sex-matched 9-month-old mice. Data are expressed as mean ± SD. *P <_ 0.01, _Cav1–/–_ versus WT or DKO (_n_ = 6–8). (**C**) Restored RV/LV+S weight ratios in DKO mice. Right and left ventricles including septum (S) were dissected free of connective tissues from age- and sex-matched mice and weighed. Data are expressed as mean ± SD. *_P <_ 0.01, _Cav1–/–_ versus WT (_n_ = 5–7); **_P <_ 0.01, DKO versus _Cav1–/–_ (_n_ = 6–8). (**D**) Restored PVR in DKO lungs. Data are expressed as mean ± SD. *_P <_ 0.01, _Cav1–/–_ versus WT; †_P <_ 0.05, _Cav1–/–_ versus DKO; **_P > 0.5, DKO versus WT (n = 4–6).
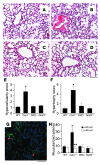
Figure 3. Prevention of pulmonary vascular remodeling induced by Cav1 deletion in DKO mice.
(A–D) Representative micrographs of H&E-stained lung sections from age- and sex-matched WT (A), Cav1–/– (B), DKO (C), and Nos3–/– (D) mice. Hypercellularity and medial thickening seen in Cav1–/– lungs were prevented in DKO lungs. Scale bar: 60 μm. (E) Histology grade score of hypercellularity in lung sections. Cellular hyperplasia was scored from 1 to 5, with 5 as the highest. Data are expressed as mean ± SD. *P <_ 0.01, _Cav1–/–_ versus WT, DKO, or eNOS (_n_ = 4–6). (**F**) Histology grade score of lung vascular hypertrophy. Medial thickness was scored from 1 to 6, with 6 being the highest. Data are expressed as mean ± SD. *_P_ < 0.01 versus WT, DKO, or eNOS (_n_ = 4–6). (**G**) Representative micrograph of immunostaining of _Cav1–/–_ lung sections with anti–α-SMA (green). Arrows show muscularized distal pulmonary arterial vessels. Scale bar: 50 μm. (**H**). Quantification of muscularized pulmonary arterial vessels in mouse lung sections. α-SMA–positive vessels were counted in 20 fields (×200) of each lung section. Data are expressed as mean ± SD. *_P <_ 0.01, _Cav1–/–_ versus WT, DKO, or eNOS (_n_ = 4–6); **_P > 0.5, Cav1–/– versus WT, DKO, or eNOS. <40 μm, vessels with diameter less than 40 μm; >40 μm, vessels with diameter greater than 40 μm.
Figure 4. Normalized ERK signaling and gene expression in DKO lungs.
(A) Western blot analysis of ERK signaling. Phosphorylation of p42/44 MAPK was detected with anti–phospho-p42/44 in lung lysates (30 μg per lane). The same blot was immunoblotted with anti-p42/44 for detection of total p42/44 and with anti–β-actin for loading control. (B–D) Quantitative analysis of gene expression in mouse lungs. Total RNA was isolated from lungs collected from 2-month-old mice, and mRNA levels of p21Cip1 (B), IGF-I (C), and VEGF-A (D) were analyzed with quantitative SYBR Green RT-PCR assay. mRNA levels of cyclophilin were used for normalization. Data are expressed as mean ± SD (n = 3–5). *P < 0.01 versus WT or DKO. Expression of p21Cip1 and growth factors IGF-I and VEGF-A was normalized in DKO lungs (similar to that in WT lungs).
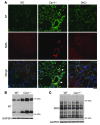
Figure 5. Increased tyrosine nitration of proteins in Cav1–/– mouse lungs.
(A) Representative micrographs of immunostaining of lung sections with anti-nitrotyrosine (NT, green) and anti–α-SMA (SMA, red). Nuclei were counterstained with DAPI (blue). Nitrotyrosine was prominent in Cav1–/– lungs, including large artery (arrowhead) and muscularized distal artery (arrow). Scale bar: 40 μm. (B) Increased protein nitration in Cav1–/– mouse lungs. Forty micrograms of lung lysates was loaded per lane. Nitrated proteins were directly detected with anti-nitrotyrosine antibody. The same blot was blotted with anti-GAPDH for loading control. We observed primarily 2 groups of proteins at molecular weights of approximately 30 and 70 kDa, respectively, with markedly increased tyrosine nitration in Cav1–/– lungs. (C) _S_-nitrosylation of proteins in Cav1–/– and WT lungs was not different. _S_-nitrosylation (SNO) in biotin-labeled lung lysates (10 μg per lane) was directly detected by Western blotting with avidin-coupled reagents.
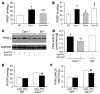
Figure 6. Impaired PKG kinase activity secondary to tyrosine nitration in Cav1–/– lungs.
(A and B) In vitro PKG activity in lung lysates during the basal state (A) and following addition of 2.5 μM cGMP (B). Data are expressed as mean ± SD (n = 3–5). *P < 0.05, Cav1–/– versus WT or DKO. (C) Prominent tyrosine nitration of PKG detected in lung lysates from Cav1–/– mice. Lung lysates (150 μg) from WT, DKO, and Cav1–/– mice were immunoprecipitated with 2 μg anti–PKG-1 antibody overnight, and PKG tyrosine nitration was detected with anti-nitrotyrosine by Western blotting. Protein expression of PKG-1 in lung lysates was also determined directly by Western blotting with anti–PKG-1 antibody. The same blot was reprobed with an antibody against mouse GAPDH as a loading control. (D) PKG-1 tyrosine nitration in cultured human pulmonary artery smooth muscle cells following SIN-1 treatment. Subconfluent primary cultures were treated with SIN-1 at the indicated concentrations for 30 minutes, and each cell lysate (50 μg) was then used for immunoprecipitation and immunoblotting for detection of PKG-1 nitration. Each lysate (15 μg) was also used for direct immunoblotting with anti–PKG-1 and anti-GAPDH. CTL, control. (E and F) SIN-1 treatment resulted in a significant decrease in PKG activity in the basal state (E) and following addition of 2.5 μM cGMP (F). Data are shown as mean ± SD (n = 3). **P < 0.001 versus CTL.
Figure 7. Identification of target tyrosine residues responsible for the impairment of PKG kinase activity upon nitration.
(A) Dose-dependent impairment of PKG activity by peroxynitrite. Purified PKG-1 was incubated with peroxynitrite at the indicated concentrations for 14 minutes at room temperature or with DETA NONOate (NONOate) for 30 minutes in the dark at room temperature. Kinase activity was then assayed. Data are expressed mean ± SD (n = 3). *P < 0.05 versus control (0 μM). (B) Screening of PKG-1α mutants with in vitro kinase assay. At 48 hours after transfection, myc-tagged WT and PKG-1α mutants were immunoprecipitated with anti-myc beads and aliquoted for incubation with either peroxynitrite (100 μM) or the same amount of 0.1N NaOH only (CTL). In vitro kinase assay was then performed to determine PKG activity. PKG activity is expressed as picomoles/minute/microgram cell lysates. Western blotting was used to detect the protein levels of PKG-1α. (C) Validation of target tyrosine residues. At 48 hours after transfection, myc-tagged WT and PKG-1α mutants were immunoprecipitated for tyrosine nitration and in vitro kinase assay. Kinase activity following peroxynitrite incubation was normalized to that of the respective control. Data are expressed as mean ± SD (n = 3). †P < 0.05 versus either PKG-1α mutant. (D) Diminished tyrosine nitration of PKG-1 mutants. After 14 minutes incubation with peroxynitrite (250 μM) at room temperature, the anti-myc immunoprecipitates were used for Western blotting to detect tyrosine nitration. The intensity of each band of PKG-1 tyrosine nitration was normalized to the intensity of the respective PKG-1 band (PKG-NT/PKG).
Figure 8. Tyrosine nitration–induced impairment of PKG activity mediates PH in Cav1–/– mice.
(A) Reduced RVSP in Cav1–/– mice treated with MnTMPyP. Cav1–/– mice were treated with either MnTMPyP (5 mg/kg, i.p., daily) (Cav1-Mn) or saline (Cav1–/–) for 6 weeks. Data are expressed as mean ± SD (n = 5 per group). *P < 0.01, Cav1–/– versus WT; **P < 0.05, Cav1-Mn versus Cav1–/– ; †P > 0.05, Cav1-Mn versus WT. (B) Inhibition of NOS with
l
-NAME reversed PH in Cav1–/– mice. Cav1–/– mice received water ad libitum (Cav1–/–, control) or water with 1 mg/ml
l
-NAME (Cav-L) or its inactive analog
d
-NAME (Cav-D) for 5 weeks. Data are expressed as mean ± SD (n = 5–7). *P < 0.01, Cav1–/– versus WT; ‡P < 0.05, Cav-L versus Cav1–/–; §P > 0.5, Cav-D versus Cav1–/–. (C–F) Restoration of PKG-1 activity in Cav1–/– lungs reversed PH. At 7 days after administration of recombinant adenovirus expressing either human PKG-1 (AdvPKG) or LacZ, lungs were collected for Western blotting (C) and PKG kinase activity assay (D) after measurements of RVSP (E) and PVR (F). PKG activity is expressed as mean ± SD (n = 3–4). #P > 0.5 versus WT; ††P < 0.05 versus WT or Cav1–/– treated with AdvPKG (D). RVSP is expressed as mean ± SD (n = 4–5). ‡‡P < 0.05, Cav1–/–-AdvPKG (PKG) versus Cav1–/–-AdvLacZ (LacZ) (E). PVR is expressed as mean ± SD (n = 3–4). §§P < 0.05, Cav1–/–-AdvPKG versus Cav1–/–-AdvLacZ (F).
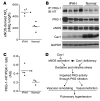
Figure 9. PKG tyrosine nitration in lung tissue from IPAH patients.
(A) Quantitative analysis of eNOS-derived NOx in human lung tissue. Following 20 minutes incubation with selective inhibitors, lung tissues were incubated with 1 mM
l
-arginine for 3 hours. eNOS-derived NOx was determined by measuring the concentration of nitrite and nitrate in the medium with the Griess reagent. Bars represent mean. *P < 0.01 versus normal. IPAH lungs produced 2-fold greater eNOS-derived NOx than normal lungs. (B) PKG tyrosine nitration in IPAH lungs. Each lysate (150 μg) was immunoprecipitated with anti–PKG-1 and immunoblotted with anti-nitrotyrosine for detection of PKG tyrosine nitration. IPAH lungs exhibited prominent PKG-1 tyrosine nitration compared with normal lungs. Protein levels of PKG-1, eNOS, and caveolin-1 were also determined by Western blotting with anti–PKG-1, -eNOS, or –caveolin-1 antibody, respectively. Immunoblot of GAPDH was used as loading control. (C) Densitometric analysis of PKG-1 tyrosine nitration. The intensity of each band of PKG-1 tyrosine nitration (PKG-1–NT) was normalized to the intensity of the respective PKG-1 band. PKG-1 tyrosine nitration increased 2-fold in IPAH lungs compared with normal lungs. Bars represent mean. *P < 0.05 (n = 3–4). (D) Proposed model of PKG nitration–mediated PH. Persistent eNOS activation secondary to caveolin-1 deficiency (Cav1–/– mice or IPAH patients) leads to formation of peroxynitrite in the pulmonary vasculature and impairment of PKG kinase activity through tyrosine nitration. Impaired PKG signaling induces pulmonary vascular remodeling and vasoconstriction, and thereby PH.
Similar articles
- A novel insight into the mechanism of pulmonary hypertension involving caveolin-1 deficiency and endothelial nitric oxide synthase activation.
Zhao YY, Malik AB. Zhao YY, et al. Trends Cardiovasc Med. 2009 Oct;19(7):238-42. doi: 10.1016/j.tcm.2010.02.003. Trends Cardiovasc Med. 2009. PMID: 20382348 Free PMC article. Review. - Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation.
Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY. Mirza MK, et al. Am J Pathol. 2010 May;176(5):2344-51. doi: 10.2353/ajpath.2010.091088. Epub 2010 Mar 19. Am J Pathol. 2010. PMID: 20304961 Free PMC article. - eNOS Activity in CAV1 Knockout Mouse Eyes.
Lei Y, Song M, Wu J, Xing C, Sun X. Lei Y, et al. Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2805-13. doi: 10.1167/iovs.15-18841. Invest Ophthalmol Vis Sci. 2016. PMID: 27228562 - Cavin-2 loss exacerbates hypoxia-induced pulmonary hypertension with excessive eNOS phosphorylation and protein nitration.
Kasahara T, Ogata T, Nakanishi N, Tomita S, Higuchi Y, Maruyama N, Hamaoka T, Matoba S. Kasahara T, et al. Heliyon. 2023 Jun 11;9(6):e17193. doi: 10.1016/j.heliyon.2023.e17193. eCollection 2023 Jun. Heliyon. 2023. PMID: 37360100 Free PMC article. - Critical Role of Caveolin-1 Loss/Dysfunction in Pulmonary Hypertension.
Mathew R. Mathew R. Med Sci (Basel). 2021 Sep 22;9(4):58. doi: 10.3390/medsci9040058. Med Sci (Basel). 2021. PMID: 34698188 Free PMC article. Review.
Cited by
- The importance of caveolin as a target in the prevention and treatment of diabetic cardiomyopathy.
Xia W, Li X, Wu Q, Xu A, Zhang L, Xia Z. Xia W, et al. Front Immunol. 2022 Nov 2;13:951381. doi: 10.3389/fimmu.2022.951381. eCollection 2022. Front Immunol. 2022. PMID: 36405687 Free PMC article. Review. - Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension.
Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Maron BA, et al. Circulation. 2012 Aug 21;126(8):963-74. doi: 10.1161/CIRCULATIONAHA.112.094722. Epub 2012 Jul 11. Circulation. 2012. PMID: 22787113 Free PMC article. - Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension.
Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, Zamanian RT, Quertermous T, Chun HJ. Chandra SM, et al. Arterioscler Thromb Vasc Biol. 2011 Apr;31(4):814-20. doi: 10.1161/ATVBAHA.110.219980. Epub 2011 Jan 13. Arterioscler Thromb Vasc Biol. 2011. PMID: 21233449 Free PMC article. - Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF.
Swärd K, Sadegh MK, Mori M, Erjefält JS, Rippe C. Swärd K, et al. Physiol Rep. 2013 Jun;1(1):e00008. doi: 10.1002/PHY2.8. Epub 2013 Jun 7. Physiol Rep. 2013. PMID: 24303100 Free PMC article. - Caveolae: A Role in Endothelial Inflammation and Mechanotransduction?
Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP. Shihata WA, et al. Front Physiol. 2016 Dec 20;7:628. doi: 10.3389/fphys.2016.00628. eCollection 2016. Front Physiol. 2016. PMID: 28066261 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases