Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse - PubMed (original) (raw)
Review
Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse
Lawrence P Carter et al. Drug Alcohol Depend. 2009.
Abstract
There are distinct differences in the accessibility, purity, dosing, and misuse associated with illicit gamma-hydroxybutyrate (GHB) compared to pharmaceutical sodium oxybate. Gamma-hydroxybutyrate sodium and sodium oxybate are the chemical and drug names, respectively, for the pharmaceutical product Xyrem (sodium oxybate) oral solution. However, the acronym GHB is also used to refer to illicit formulations that are used for non-medical purposes. This review highlights important differences between illicit GHB and sodium oxybate with regard to their relative abuse liability, which includes the likelihood and consequences of abuse. Data are summarized from the scientific literature; from national surveillance systems in the U.S., Europe, and Australia (for illicit GHB); and from clinical trials and post-marketing surveillance with sodium oxybate (Xyrem). In the U.S., the prevalence of illicit GHB use, abuse, intoxication, and overdose has declined from 2000, the year that GHB was scheduled, to the present and is lower than that of most other licit and illicit drugs. Abuse and misuse of the pharmaceutical product, sodium oxybate, has been rare over the 5 years since its introduction to the market, which is likely due in part to the risk management program associated with this product. Differences in the accessibility, purity, dosing, and misuse of illicit GHB and sodium oxybate suggest that risks associated with illicit GHB are greater than those associated with the pharmaceutical product sodium oxybate.
Figures
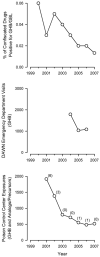
Figure 1
National estimates of the availability, use, and abuse of GHB in the U.S. Top panel: Percent of items confiscated in law enforcement operations in the U.S. from 2000 through 2007 that were positive for GHB or GBL. Data are from the annual reports from The National Forensic Laboratory Information System (NFLIS). Data are not available before the year 2000 because GHB was not illegal until 2000. Middle panel: Number of emergency department visits in which GHB was mentioned. Data are from the annual reports from the Drug Abuse Warning Network (DAWN) coordinated by the Substance Abuse and Mental Health Services Administration (SAMHSA) of the U.S. Department of Health and Human Services. Note: trends prior to 2003 are not shown due to methodological changes made to the DAWN surveillance system that preclude the comparison of DAWN data from before and after 2003. Bottom panel: Number of exposures (circles) and deaths (numbers in parentheses) attributed to illicit GHB, GHB analogs, and GHB precursors in each of the following years. Data are from the Annual Reports from the American Association of Poison Control Centers National Poison Database (formerly named the Toxic Exposure Surveillance System).
Similar articles
- The Xyrem risk management program.
Fuller DE, Hornfeldt CS, Kelloway JS, Stahl PJ, Anderson TF. Fuller DE, et al. Drug Saf. 2004;27(5):293-306. doi: 10.2165/00002018-200427050-00002. Drug Saf. 2004. PMID: 15061684 Review. - The clinical development of gamma-hydroxybutyrate (GHB).
Wedin GP, Hornfeldt CS, Ylitalo LM. Wedin GP, et al. Curr Drug Saf. 2006 Jan;1(1):99-106. doi: 10.2174/157488606775252647. Curr Drug Saf. 2006. PMID: 18690919 Review. - From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy.
Fuller DE, Hornfeldt CS. Fuller DE, et al. Pharmacotherapy. 2003 Sep;23(9):1205-9. doi: 10.1592/phco.23.10.1205.32756. Pharmacotherapy. 2003. PMID: 14524654 Clinical Trial. - Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion.
Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Wang YG, et al. J Clin Sleep Med. 2009 Aug 15;5(4):365-71. J Clin Sleep Med. 2009. PMID: 19968016 Free PMC article. Review. - Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome.
Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S. Busardò FP, et al. Eur Rev Med Pharmacol Sci. 2015 Dec;19(23):4654-63. Eur Rev Med Pharmacol Sci. 2015. PMID: 26698265 Review.
Cited by
- Physical dependence on gamma-hydroxybutrate (GHB) prodrug 1,4-butanediol (1,4-BD): time course and severity of withdrawal in baboons.
Goodwin AK, Gibson KM, Weerts EM. Goodwin AK, et al. Drug Alcohol Depend. 2013 Oct 1;132(3):427-33. doi: 10.1016/j.drugalcdep.2013.02.035. Epub 2013 Mar 26. Drug Alcohol Depend. 2013. PMID: 23538206 Free PMC article. - Dosing Optimization of Low-Sodium Oxybate in Narcolepsy and Idiopathic Hypersomnia in Adults: Consensus Recommendations.
Morse AM, Bogan RK, Roy A, Thorpy MJ. Morse AM, et al. Neurol Ther. 2024 Jun;13(3):785-807. doi: 10.1007/s40120-024-00607-8. Epub 2024 Apr 25. Neurol Ther. 2024. PMID: 38662324 Free PMC article. - γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology.
Felmlee MA, Morse BL, Morris ME. Felmlee MA, et al. AAPS J. 2021 Jan 8;23(1):22. doi: 10.1208/s12248-020-00543-z. AAPS J. 2021. PMID: 33417072 Free PMC article. Review. - Efficacy and Safety of Sodium Oxybate in Isolated Focal Laryngeal Dystonia: A Phase IIb Double-Blind Placebo-Controlled Cross-Over Randomized Clinical Trial.
Simonyan K, O'Flynn LC, Hamzehei Sichani A, Frucht SJ, Rumbach AF, Sharma N, Song PC, Worthley A. Simonyan K, et al. Ann Neurol. 2025 Feb;97(2):329-343. doi: 10.1002/ana.27121. Epub 2024 Nov 20. Ann Neurol. 2025. PMID: 39565101 Free PMC article. Clinical Trial. - Gamma-hydroxybutyrate abuse: pharmacology and poisoning and withdrawal management.
Marinelli E, Beck R, Malvasi A, Lo Faro AF, Zaami S. Marinelli E, et al. Arh Hig Rada Toksikol. 2020 Mar 1;71(1):19-26. doi: 10.2478/aiht-2020-71-3314. Arh Hig Rada Toksikol. 2020. PMID: 32597141 Free PMC article. Review.
References
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, Pastor A, de la Torre R. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. J Clin Psychopharmacol. 2007;27:625–638. - PubMed
- Abanades S, Farré M, Segura M, Pichini S, Barral D, Pacifici R, Pellegrini M, Fonseca F, Langohr K, De La Torre R. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Ann N Y Acad Sci. 2006;1074:559–576. - PubMed
- Arfken CL, Schuster CR, Johanson CE. Postmarketing surveillance of abuse liability of sibutramine. Drug Alcohol Depend. 2003;69:169–173. - PubMed
- Australian Institute of Health and Welfare. Canberra: AIHW; 2008. [Oct 8, 2008]. 2007 National Drug Strategy Household Survey: first results. Drug Statistics Series number 20. Cat. no. PHE 98. http://www.aihw.gov.au/publications/index.cfm/title/10579.