Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia - PubMed (original) (raw)
Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia
Yumi Imai et al. J Virol. 2009 Aug.
Abstract
We previously demonstrated that herpes simplex virus type 1 (HSV-1) preferentially establishes latent infection in monoclonal antibody (MAb) A5-positive ganglionic neurons and that a 2.8-kb portion of the HSV-1 genome, corresponding to the 5' end of the LAT (latency-associated transcript) coding region, is responsible for this phenotype (38, 65). In the current study we carried out further genetic mapping of this latency phenotype and investigated some of the mechanisms that might be responsible. Studies with the chimeric virus HSV-1 17syn+/LAT2, an HSV-1 virus engineered to express HSV-2 LAT, demonstrated that this virus exhibited an HSV-2 latency phenotype, preferentially establishing latency in MAb KH10-positive neurons. This result is complementary to that previously described for the chimeric virus HSV-2 333/LAT1 and indicate that the HSV-1 latency phenotype can be changed to that of HSV-2 by substitution of a 2.8-kb piece of complementary viral DNA. Sequential studies in which we evaluated the pattern of HSV-1 latent infection of the mouse trigeminal ganglion following ocular inoculation with viruses with deletions of functional thymidine kinase, glycoprotein E, ICP0, and US9 protein demonstrate that preferential establishment of HSV-1 latent infection in A5-positive neurons is not a consequence of (i) differential access of HSV-1 to A5-positive neurons,(ii) differential cell-to-cell spread of HSV-1 to A5-positive neurons, (iii) differential "round-trip" spread of HSV-1 to A5-positive neurons, or (iv) expression of ICP0. Additional mapping studies with the HSV-1 LAT deletion viruses dLAT371, 17DeltaSty, and 17Delta348 indicate that most of the LAT 5' exon is not required for HSV-1 to preferentially establish latent infection in A5-positive neurons.
Figures
FIG. 1.
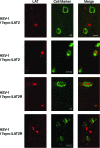
Identification of TG neurons latently infected with 17syn+/LAT2 or 17syn+/LAT2R. Sections of murine TG were assayed by both in situ hybridization for LAT (red-labeled nuclei) and IF staining with MAbs A5 and KH10 (green-labeled cell bodies) in order to identify the neuronal subpopulations latently infected with either a chimeric virus or its rescuant. HSV-2-specific probes were used to detect LAT expression in 17syn+/LAT2-infected tissue, and HSV-1-specific probes were used to detect LAT expression in 17syn+/LAT2R-infected tissue. In these representative images, LAT expression of 17syn+/LAT2 is colocalized with a KH10-positive neuron but not with A5-positive neurons. In contrast, LAT expression of 17syn+/LAT2R is colocalized with an A5-positive neuron but not with KH10-positive neurons. Bar, 20 μm.
FIG. 2.
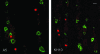
Identification of TG neurons latently infected with _tk_LTRZ1. Sections of murine TG were assayed by both in situ hybridization for LAT (red-labeled nuclei) and IF staining with MAbs A5 (left) and KH10 (right) to identify the neuronal subpopulations (green-labeled cell bodies) latently infected with _tk_LTRZ1. In these representative images, LAT expression does not colocalize with either A5- or KH10-positive neurons. Bar, 20 μm.
FIG. 3.
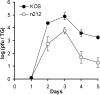
Viral titers of TG acutely infected with KOS and _n_212. For each time point after ocular inoculation, TG from five mice were assayed for infectious virus by standard plaque assay. Data are presented as mean and standard error of the mean per mouse.
Similar articles
- Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts.
Margolis TP, Imai Y, Yang L, Vallas V, Krause PR. Margolis TP, et al. J Virol. 2007 Feb;81(4):1872-8. doi: 10.1128/JVI.02110-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151134 Free PMC article. - LAT region factors mediating differential neuronal tropism of HSV-1 and HSV-2 do not act in trans.
Bertke AS, Apakupakul K, Ma A, Imai Y, Gussow AM, Wang K, Cohen JI, Bloom DC, Margolis TP. Bertke AS, et al. PLoS One. 2012;7(12):e53281. doi: 10.1371/journal.pone.0053281. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300908 Free PMC article. - Herpes Simplex Virus 1 Infection of Tree Shrews Differs from That of Mice in the Severity of Acute Infection and Viral Transcription in the Peripheral Nervous System.
Li L, Li Z, Wang E, Yang R, Xiao Y, Han H, Lang F, Li X, Xia Y, Gao F, Li Q, Fraser NW, Zhou J. Li L, et al. J Virol. 2015 Oct 28;90(2):790-804. doi: 10.1128/JVI.02258-15. Print 2016 Jan 15. J Virol. 2015. PMID: 26512084 Free PMC article. - Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival.
Thompson RL, Sawtell NM. Thompson RL, et al. J Virol. 2001 Jul;75(14):6660-75. doi: 10.1128/JVI.75.14.6660-6675.2001. J Virol. 2001. PMID: 11413333 Free PMC article. - Herpes Simplex Virus 1 MicroRNA miR-H8 Is Dispensable for Latency and Reactivation In Vivo.
Barrozo ER, Nakayama S, Singh P, Neumann DM, Bloom DC. Barrozo ER, et al. J Virol. 2021 Jan 28;95(4):e02179-20. doi: 10.1128/JVI.02179-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33208453 Free PMC article.
Cited by
- Herpes Simplex Virus 2 Counteracts Neurite Outgrowth Repulsion during Infection in a Nerve Growth Factor-Dependent Manner.
Kropp KA, López-Muñoz AD, Ritter B, Martín R, Rastrojo A, Srivaratharajan S, Döhner K, Dhingra A, Czechowicz JS, Nagel CH, Sodeik B, Alcami A, Viejo-Borbolla A. Kropp KA, et al. J Virol. 2020 Sep 29;94(20):e01370-20. doi: 10.1128/JVI.01370-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32669337 Free PMC article. - Resident T Cells Are Unable To Control Herpes Simplex Virus-1 Activity in the Brain Ependymal Region during Latency.
Menendez CM, Jinkins JK, Carr DJ. Menendez CM, et al. J Immunol. 2016 Aug 15;197(4):1262-75. doi: 10.4049/jimmunol.1600207. Epub 2016 Jun 29. J Immunol. 2016. PMID: 27357149 Free PMC article. - Tegument protein control of latent herpesvirus establishment and animation.
Penkert RR, Kalejta RF. Penkert RR, et al. Herpesviridae. 2011 Feb 8;2(1):3. doi: 10.1186/2042-4280-2-3. Herpesviridae. 2011. PMID: 21429246 Free PMC article. - A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.
Kennedy PG, Rovnak J, Badani H, Cohrs RJ. Kennedy PG, et al. J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review. - Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons.
Bertke AS, Ma A, Margolis MS, Margolis TP. Bertke AS, et al. J Virol. 2013 Jun;87(11):6512-6. doi: 10.1128/JVI.00383-13. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514893 Free PMC article.
References
- Atanasiu, D., J. R. Kent, J. J. Gartner, and N. W. Fraser. 2006. The stable 2-kb LAT intron of herpes simplex stimulates the expression of heat shock proteins and protects cells from stress. Virology 35026-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY10008/EY/NEI NIH HHS/United States
- R21 AI081072/AI/NIAID NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
- R01 EY010008/EY/NEI NIH HHS/United States
- EY02162/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous