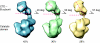3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases - PubMed (original) (raw)
3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases
Sebastian Klinge et al. EMBO J. 2009.
Abstract
Eukaryotic DNA replication requires the coordinated activity of the multi-subunit DNA polymerases: Pol alpha, Pol delta and Pol epsilon. The conserved catalytic and regulatory B subunits associate in a constitutive heterodimer that represents the functional core of all three replicative polymerases. Here, we combine X-ray crystallography and electron microscopy (EM) to describe subunit interaction and 3D architecture of heterodimeric yeast Pol alpha. The crystal structure of the C-terminal domain (CTD) of the catalytic subunit bound to the B subunit illustrates a conserved mechanism of accessory factor recruitment by replicative polymerases. The EM reconstructions of Pol alpha reveal a bilobal shape with separate catalytic and regulatory modules. Docking of the B-CTD complex in the EM reconstruction shows that the B subunit is tethered to the polymerase domain through a structured but flexible linker. Our combined findings provide a structural template for the common functional architecture of the three major replicative DNA polymerases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
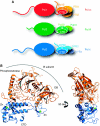
Crystal structure of the yeast Pol α CTD–B subunit complex. (A) The eukaryotic DNA polymerases α, δ and ɛ are multi-subunit enzymes. The drawing shows the subunit organisation of yeast Pol α, Pol δ and Pol ɛ. A heterodimeric complex of catalytic subunit and accessory B subunit forms their common functional core. The interaction is mediated by the conserved C-terminal domain of the catalytic subunit. The extended intervening sequence between polymerase domain and CTD in the catalytic subunit of Pol ɛ is not drawn to scale. (B) Ribbon diagram of the crystal structure of the yeast Pol α CTD–B subunit complex, showing two orientations of the complex related by a 90 ° rotation. The CTD is drawn in blue and the B subunit in orange. The position of the phosphoesterase and OB domains in the B subunit are indicated. The zinc atoms are shown as green spheres.
Figure 2
The yeast Pol α CTD. (A) Tube diagram of the CTD, coloured according to secondary structure (red: α helices; green: β strand; wheat: irregular coil). The zinc atoms are drawn as cyan spheres. The position of the two zinc-binding modules, Zn-1 and Zn-2, are indicated by circles. (B) Superposition of the Zn-1 and Zn-2 modules of CTD. The side chains of the cysteine ligands are drawn as sticks and the polypeptide chains are coloured blue (N-terminus) to red (C-terminus). (C) Multiple sequence alignment of the CTD region of Pol α from distantly related eukaryotic species (Sce, Saccharomyces cerevisiae; Dme, Drosophila melanogaster; Xla, Xenopus laevis; Gga, Gallus gallus; Hsa, Homo sapiens). Completely conserved residues are shown in white in blue background, identical residues in yellow and conserved residues in cyan. The elements of secondary structure as observed in the crystal structure of the complex are drawn above the alignment (α helices as cylinders and β strands as arrows). The cysteine ligands that coordinate the zinc atoms are boxed in red, and the extent of Zn-1 and Zn-2 modules is marked under the alignment. Residues at the interface with the B subunit are indicated by an asterisk.
Figure 3
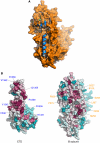
The interface of the CTD–B subunit complex. (A) Overall view highlighting the involvement of CTD helix α2 and the Zn-2 module in the interaction with the B subunit. The structural elements of the CTD are depicted as blue ribbons, the B subunit as a molecular surface in orange. (B) Evolutionary conservation of surface amino acids mapped on the Pol α CTD and B subunit structures in space-fill representation, calculated with ConSurf (Landau et al, 2005) from an alignment of 50 Pol α CTD and 66 B subunit sequences. Amino-acid conservation is indicated by a transition in colour hues, from magenta (most conserved) to cyan (most variable). The position of some of the strongly conserved CTD and B subunit residues at the interface is indicated.
Figure 4
Details of subunit interactions at the CTD–B interface. (A) Close-up views of hydrophilic interactions at the CTD α2–B subunit interface. Side chains are drawn as sticks, water molecules as cyan spheres and hydrogen bonds as dashed yellow lines. (B) Hydrophobic interactions at the CTD Zn-2–B subunit interface. CTD side chains are represented as in panel A. The molecular surface of the B subunit is shown in orange. (C) Topology diagram of the CTD structure. The α helices are shown as red cylinders and the β strands as green arrows. A sequence alignment for CTD helix α2 of yeast Pol α, Pol δ and Pol ɛ is shown below the diagram; identical residues are highlighted in black and conserved residues in grey.
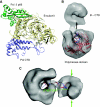
Figure 5
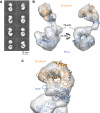
3D reconstruction of heterodimeric yeast Pol α using electron microscopy. (A) Reference-free 2D averages obtained from images of the heterodimeric Pol α reveal the presence of two distinct regions in the structure. (B) Ribbon models for the crystal structures of the CTD–B complex and the catalytic domain of the archaeal polymerase from T. gorgonarius (PDB id 2VWJ) are fitted to the EM reconstruction. Two rotated views of the EM reconstruction are shown, represented as white transparent density. The Pol α CTD and the catalytic domain of the archaeal polymerase are shown in blue and the B subunit in orange. (C) Close-up view of the EM reconstruction of Pol α, demonstrating the fitting of the CTD–B complex within the smaller lobe of the EM reconstruction.
Figure 6
Conformational flexibility of Pol α. The EM reconstructions highlight the variability in the respective position of the lobes containing catalytic and B subunits. The structural heterogeneity is illustrated by a representative view of the three reconstructions obtained. The plasticity of the ‘arm' region suggests that controlled changes in the relative position of the two subunits might be relevant to the function of Pol α. The percentage values reported under the EM reconstructions reflect the proportion of particles assigned to the three 3D solutions and represents an estimate of the prevalence of each conformation in the data set.
Figure 7
Functional interactions of the heterodimeric Pol α. (A) Superposition of B subunits of the CTD–B complex of yeast Pol α and the p50-p66 complex (PDB id 3E0J) of human Pol δ. The superposition illustrates how the B subunit of replicative polymerases can act as a structural scaffold by engaging in multiple, concomitant protein–protein interactions. The protein chains are drawn as ribbons, coloured pale yellow for the B subunits, blue for the Pol α CTD and green for Pol δ p66. (B) The position of the DNA molecule bound to the T. gorgonarius polymerase (PDB id 2VWJ) fitted to the EM reconstruction suggests a possible trajectory for the nascent DNA emerging from the active site of Pol α. (C) Proposed model for the interaction of Pol α with DNA. An idealised B-form DNA molecule spanning two helical turns has been modelled within Pol α, to span the distance between active site and the smaller lobe containing the B subunit. The green arrows highlight possible sites on the surface of the smaller lobe that might be involved in the interaction with the primase subunits.
Similar articles
- Crystal Structure of the Human Pol α B Subunit in Complex with the C-terminal Domain of the Catalytic Subunit.
Suwa Y, Gu J, Baranovskiy AG, Babayeva ND, Pavlov YI, Tahirov TH. Suwa Y, et al. J Biol Chem. 2015 Jun 5;290(23):14328-37. doi: 10.1074/jbc.M115.649954. Epub 2015 Apr 6. J Biol Chem. 2015. PMID: 25847248 Free PMC article. - A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome.
Simon AC, Zhou JC, Perera RL, van Deursen F, Evrin C, Ivanova ME, Kilkenny ML, Renault L, Kjaer S, Matak-Vinković D, Labib K, Costa A, Pellegrini L. Simon AC, et al. Nature. 2014 Jun 12;510(7504):293-297. doi: 10.1038/nature13234. Epub 2014 May 4. Nature. 2014. PMID: 24805245 Free PMC article. - Crystal structure of the C-terminal domain of human DNA primase large subunit: implications for the mechanism of the primase-polymerase α switch.
Agarkar VB, Babayeva ND, Pavlov YI, Tahirov TH. Agarkar VB, et al. Cell Cycle. 2011 Mar 15;10(6):926-31. doi: 10.4161/cc.10.6.15010. Epub 2011 Mar 15. Cell Cycle. 2011. PMID: 21346410 Free PMC article. - New Insights into the Mechanism of DNA Duplication by the Eukaryotic Replisome.
Pellegrini L, Costa A. Pellegrini L, et al. Trends Biochem Sci. 2016 Oct;41(10):859-871. doi: 10.1016/j.tibs.2016.07.011. Epub 2016 Aug 20. Trends Biochem Sci. 2016. PMID: 27555051 Review. - Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes.
Brautigam CA, Steitz TA. Brautigam CA, et al. Curr Opin Struct Biol. 1998 Feb;8(1):54-63. doi: 10.1016/s0959-440x(98)80010-9. Curr Opin Struct Biol. 1998. PMID: 9519297 Review.
Cited by
- A conserved motif in the C-terminal tail of DNA polymerase α tethers primase to the eukaryotic replisome.
Kilkenny ML, De Piccoli G, Perera RL, Labib K, Pellegrini L. Kilkenny ML, et al. J Biol Chem. 2012 Jul 6;287(28):23740-7. doi: 10.1074/jbc.M112.368951. Epub 2012 May 16. J Biol Chem. 2012. PMID: 22593576 Free PMC article. - Iron-Sulfur Clusters in DNA Polymerases and Primases of Eukaryotes.
Baranovskiy AG, Siebler HM, Pavlov YI, Tahirov TH. Baranovskiy AG, et al. Methods Enzymol. 2018;599:1-20. doi: 10.1016/bs.mie.2017.09.003. Epub 2017 Dec 6. Methods Enzymol. 2018. PMID: 29746236 Free PMC article. - The architecture of yeast DNA polymerase ζ.
Gómez-Llorente Y, Malik R, Jain R, Choudhury JR, Johnson RE, Prakash L, Prakash S, Ubarretxena-Belandia I, Aggarwal AK. Gómez-Llorente Y, et al. Cell Rep. 2013 Oct 17;5(1):79-86. doi: 10.1016/j.celrep.2013.08.046. Epub 2013 Oct 10. Cell Rep. 2013. PMID: 24120860 Free PMC article. - The CDC13-STN1-TEN1 complex stimulates Pol α activity by promoting RNA priming and primase-to-polymerase switch.
Lue NF, Chan J, Wright WE, Hurwitz J. Lue NF, et al. Nat Commun. 2014 Dec 12;5:5762. doi: 10.1038/ncomms6762. Nat Commun. 2014. PMID: 25503194 Free PMC article. - Structural basis for the interaction of a hexameric replicative helicase with the regulatory subunit of human DNA polymerase α-primase.
Zhou B, Arnett DR, Yu X, Brewster A, Sowd GA, Xie CL, Vila S, Gai D, Fanning E, Chen XS. Zhou B, et al. J Biol Chem. 2012 Aug 3;287(32):26854-66. doi: 10.1074/jbc.M112.363655. Epub 2012 Jun 14. J Biol Chem. 2012. PMID: 22700977 Free PMC article.
References
- Asturias FJ, Cheung IK, Sabouri N, Chilkova O, Wepplo D, Johansson E (2006) Structure of Saccharomyces cerevisiae DNA polymerase epsilon by cryo-electron microscopy. Nat Struct Mol Biol 13: 35–43 - PubMed
- Berger I, Fitzgerald DJ, Richmond TJ (2004) Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol 22: 1583–1587 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases