Structure of a mitochondrial ribosome with minimal RNA - PubMed (original) (raw)
Structure of a mitochondrial ribosome with minimal RNA
Manjuli R Sharma et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The Leishmania tarentolae mitochondrial ribosome (Lmr) is a minimal ribosomal RNA (rRNA)-containing ribosome. We have obtained a cryo-EM map of the Lmr. The map reveals several features that have not been seen in previously-determined structures of eubacterial or eukaryotic (cytoplasmic or organellar) ribosomes to our knowledge. Comparisons of the Lmr map with X-ray crystallographic and cryo-EM maps of the eubacterial ribosomes and a cryo-EM map of the mammalian mitochondrial ribosome show that (i) the overall structure of the Lmr is considerably more porous, (ii) the topology of the intersubunit space is significantly different, with fewer intersubunit bridges, but more tunnels, and (iii) several of the functionally-important rRNA regions, including the alpha-sarcin-ricin loop, have different relative positions within the structure. Furthermore, the major portions of the mRNA channel, the tRNA passage, and the nascent polypeptide exit tunnel contain Lmr-specific proteins, suggesting that the mechanisms for mRNA recruitment, tRNA interaction, and exiting of the nascent polypeptide in Lmr must differ markedly from the mechanisms deduced for ribosomes in other organisms. Our study identifies certain structural features that are characteristic solely of mitochondrial ribosomes and other features that are characteristic of both mitochondrial and chloroplast ribosomes (i.e., organellar ribosomes).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
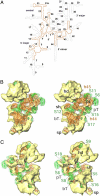
Cryo-EM map of the Lmr (A and D) and its side-by-side comparison with the maps of an eubacterial (E. coli) 70S ribosome (18) (B and E) and a mammalian (Bos taurus) 55S mitoribosome (20) (C and F). Maps are shown from the L7/L12-stalk (A–C) and the protein L1 (D–F) sides. The SSU (yellow) and LSU (blue) are depicted. Numbers (1–9 in A and D) point to a few obvious differences in the Lmr: 1, gap caused by absence of major portion of SSU rRNA helix 44 (see Fig. 2_A_); 2 and 3, larger gaps between the head (h) of SSU and central protuberance (CP) of LSU; 4, an extended structure emerging from the CP; and 5–9, gaps caused by absence of various rRNA segments in the Lmr. Landmarks of the SSU are: b, body; mgt, mRNA gate (in C and F); pt, platform; sh, shoulder; sp, spur. Landmarks of the LSU are: L1, L1 protein (corresponding density is marked by * in D); Sb, L7/L12 stalk base; FBR, factor binding region. The scale bar is shown in D.
Fig. 2.
Structural analysis of the SSU. (A) Secondary-structure line diagram of the 9S Lm-rRNA (orange), superimposed on that of the bacterial 16S rRNA (13) (gray). RNA regions absent in the Lmr thus appear gray. RNA helices are identified by the adjacent numbers. Dashed lines correspond to unassigned segments of the 9S Lm-rRNA. (B) Stereo representation of the fitting of the conserved domains (ribbons) into the cryo-EM map of the SSU, shown from its interface side. The conserved components (orange, rRNA; green, proteins) are shown as translucent surfaces, and the Lmr-specific proteins (yellow) are shown as solid surfaces. (C) Same as B but shown from a solvent-side. Landmarks: numbers prefixed with S represent the SSU proteins; numbers prefixed with h identify the 9S rRNA helices; hd, head; bT, body tunnel; pT, platform tunnel. All other landmarks are as in Fig. 1.
Fig. 3.
Structural analysis of the LSU. (A) Secondary-structure diagram of the 12S Lm-rRNA (purple), superimposed on that of the bacterial 23S rRNA (14) (gray). Roman numerals identify the 6 domains of the rRNA, and helices are identified by numbers. Dashed lines correspond to unassigned segments of the 12S Lm-rRNA. (B) Stereo representation of the fitting of conserved domains (ribbons) into the cryo-EM map of the LSU, shown from its interface side. The conserved components (pinkish purple, rRNA; green, proteins) are shown as translucent surfaces, and the Lmr-specific proteins (blue) are shown as solid surfaces. (C) Same as B, but shown from the solvent side. Landmarks: numbers prefixed with L identify the LSU proteins; numbers prefixed with H identify the LSU rRNA helices; APSF, A- and P-site finger; CPT, CP tunnel; L9-N, N-terminal domain of L9. All other landmarks are as in Fig. 1.
Fig. 4.
Locations of intersubunit bridges. The SSU (A) and LSU (B) are shown from their interface sides. Bridges have been marked on both subunits as red ellipses and circles. Color codes for the SSU and LSU components are the same as in Figs. 2 and 3.
Fig. 5.
Stereo representation of topology of the mRNA and tRNA paths on the Lmr. (A) The tRNA path from A/T state to A site on the LSU. (B) The mRNA and tRNA paths encompassing A and P sites on the SSU. Positions of the A/T state (A/T, orange) and the A-site (A, pink) tRNAs are adopted from ref. , and the position of P-site tRNA (P, green) is adopted from ref. . The Lmr-specific protein densities (blue and yellow, respectively, in A and B) are marked by *. Landmarks: AC and CCA, anticodon and acceptor ends, respectively, of tRNAs. All other landmarks are as in previous figures.
Fig. 6.
Topology of the polypeptide-exit tunnel. (A) Cut-away view of the LSU (white surfaces correspond to the cutting plane) to reveal the tunnel. (B) The PES, as seen from the bottom of the LSU. (C) The LSU is shown from the side opposite to that in A to reveal the PAS. A model of an α-helical polypeptide chain (red) is used to delineate the tunnel (35). * in A points to location of PAS that lies behind the cutting plane. All other landmarks are as in previous figures. The orientations of the Lmr, with the area boxed in red in the thumbnail enlarged, are shown in the corresponding panels to the left.
Similar articles
- Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome.
Kaushal PS, Sharma MR, Booth TM, Haque EM, Tung CS, Sanbonmatsu KY, Spremulli LL, Agrawal RK. Kaushal PS, et al. Proc Natl Acad Sci U S A. 2014 May 20;111(20):7284-9. doi: 10.1073/pnas.1401657111. Epub 2014 May 5. Proc Natl Acad Sci U S A. 2014. PMID: 24799711 Free PMC article. - Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins.
Sharma MR, Koc EC, Datta PP, Booth TM, Spremulli LL, Agrawal RK. Sharma MR, et al. Cell. 2003 Oct 3;115(1):97-108. doi: 10.1016/s0092-8674(03)00762-1. Cell. 2003. PMID: 14532006 - Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation.
Spahn CM, Gomez-Lorenzo MG, Grassucci RA, Jørgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP, Frank J. Spahn CM, et al. EMBO J. 2004 Mar 10;23(5):1008-19. doi: 10.1038/sj.emboj.7600102. Epub 2004 Feb 19. EMBO J. 2004. PMID: 14976550 Free PMC article. - The 55S mammalian mitochondrial ribosome and its tRNA-exit region.
Kaushal PS, Sharma MR, Agrawal RK. Kaushal PS, et al. Biochimie. 2015 Jul;114:119-26. doi: 10.1016/j.biochi.2015.03.013. Epub 2015 Mar 20. Biochimie. 2015. PMID: 25797916 Free PMC article. Review. - Joachim Frank's Binding with the Ribosome.
Li W, Agrawal RK. Li W, et al. Structure. 2019 Mar 5;27(3):411-419. doi: 10.1016/j.str.2018.11.006. Epub 2018 Dec 27. Structure. 2019. PMID: 30595455 Free PMC article. Review.
Cited by
- Pentatricopeptide repeat proteins stimulate mRNA adenylation/uridylation to activate mitochondrial translation in trypanosomes.
Aphasizheva I, Maslov D, Wang X, Huang L, Aphasizhev R. Aphasizheva I, et al. Mol Cell. 2011 Apr 8;42(1):106-17. doi: 10.1016/j.molcel.2011.02.021. Mol Cell. 2011. PMID: 21474072 Free PMC article. - Extreme mitochondrial evolution in the ctenophore Mnemiopsis leidyi: Insight from mtDNA and the nuclear genome.
Pett W, Ryan JF, Pang K, Mullikin JC, Martindale MQ, Baxevanis AD, Lavrov DV. Pett W, et al. Mitochondrial DNA. 2011 Aug;22(4):130-42. doi: 10.3109/19401736.2011.624611. Epub 2011 Oct 10. Mitochondrial DNA. 2011. PMID: 21985407 Free PMC article. - Cbp3-Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly.
Gruschke S, Kehrein K, Römpler K, Gröne K, Israel L, Imhof A, Herrmann JM, Ott M. Gruschke S, et al. J Cell Biol. 2011 Jun 13;193(6):1101-14. doi: 10.1083/jcb.201103132. J Cell Biol. 2011. PMID: 21670217 Free PMC article. - Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome.
Kaushal PS, Sharma MR, Booth TM, Haque EM, Tung CS, Sanbonmatsu KY, Spremulli LL, Agrawal RK. Kaushal PS, et al. Proc Natl Acad Sci U S A. 2014 May 20;111(20):7284-9. doi: 10.1073/pnas.1401657111. Epub 2014 May 5. Proc Natl Acad Sci U S A. 2014. PMID: 24799711 Free PMC article. - The tRNA Elbow in Structure, Recognition and Evolution.
Zhang J, Ferré-D'Amaré AR. Zhang J, et al. Life (Basel). 2016 Jan 12;6(1):3. doi: 10.3390/life6010003. Life (Basel). 2016. PMID: 26771646 Free PMC article. Review.
References
- Vickerman K, Preston TM. In: Biology of the Kinetoplastida. Lumsden WHR, Evans DA, editors. London: Academic; 1976. pp. 35–130.
- Lukeš J, Hashimi H, Zíková A. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr Genet. 2005;48:277–299. - PubMed
- Stuart KD, Schnaufer A, Ernst NL, Panigrahi A. Complex management: RNA editing in trypanosomes. Trends Biochem Sci. 2005;30:97–105. - PubMed
- Horváth A, Neboháčová M, Lukeš J, Maslov DA. Unusual polypeptide synthesis in the kinetoplast-mitochondria from Leishmania tarentolae. Identification of individual de novo translation products. J Biol Chem. 2002;277:7222–7230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources