Nef proteins from simian immunodeficiency viruses are tetherin antagonists - PubMed (original) (raw)
Nef proteins from simian immunodeficiency viruses are tetherin antagonists
Fengwen Zhang et al. Cell Host Microbe. 2009.
Abstract
The tetherin/BST2/CD317 protein blocks the release of HIV-1 and other enveloped viruses by inducing tethering of nascent particles to infected cell surfaces. The HIV-1 Vpu protein antagonizes the antiviral activity of human but not monkey tetherins and many simian immunodeficiency viruses (SIVs) do not encode Vpu. Here, we show that the apparently "missing" antitetherin activity in SIVs has been acquired by several SIV Nef proteins. Specifically, SIV(MAC)/SIV(SMM), SIV(AGM), and SIV(BLU) Nef proteins can suppress tetherin activity. Notably, tetherin antagonism by SIV Nef proteins is species specific, is genetically separable from other Nef activities, and is most evident with simian rather than human tetherin proteins. Accordingly, a critical determinant of sensitivity to SIV(MAC) Nef in the tetherin cytoplasmic tail is variable in nonhuman primate tetherins and deleted in human tetherin, likely due to selective pressures imposed by viral antagonists, perhaps including Nef proteins.
Figures
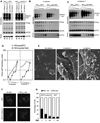
Figure 1. Effects of Env and Nef proteins on SIVMAC239 particle release
(A) Western blot analysis of wildtype (WT) and envelope deleted (del Env) SIVMAC239 particle release in the presence of tetherin-HA proteins from rhesus (rh) and pig-tailed macaques (pgt). Cells were transfected with 400ng of proviral plasmid and 40ng of each tetherin expression plasmid. Cell and virion lysates were probed with an anti-capsid monoclonal and/or rabbit anti-HA antibody, and signals detected using fluorescent secondary antibodies. The numbers below each lane indicate quantitation (LICOR) of the p27CA present in the virion pellet. (B,C) Western blot analysis of wildtype (WT) and Nef-deleted (del Nef) SIVMAC239 particle release in the presence of the hu-tetherin (B) or rh-tetherin-1 (C) proteins. Cells were transfected with a fixed amount (400ng) of proviral plasmid and varying amounts (50, 25, 12.5, 6.25, 3.12 or 0ng) of each tetherin expression plasmid. Cell and virions lysates were probed with an anti-capsid and anti-HA epitope monoclonal antibodies, and signals detected using chemiluminescence reagents. The results shown are representative of at least three separate experiments. (D) Quantitation of virion release inhibition by tetherin. P27CA in virion samples from panel (B) and (C) was measured using quantitative fluorescence-based western blotting (LICOR). (E) Representative scanning EM images of cells transfected with pSIVGag-IRESGFP and either no tetherin, rh-tetherin-1 or hu-tetherin. Cells were selected for imaging based on similar levels of GFP fluorescence. Scale bar indicates 2µm. (F) Representative fluorescence micrographs of unmanipulated or rh-tetherin-1 expressing 293T cells transfected with plasmids expressing SIVMAC239 Gag-GFP fusion protein, in the presence or absence of SIVMAC239 Nef. (G) Quantitation of the proportion of cells displaying both plasma membrane and internal (PM+Int) or plasma membrane only (PM) intense accumulations of SIVMAC239 Gag-GFP, under the same experimental conditions as in (F) (see (F) for examples). Cells were transfected with 0µg (−), 0.8µg(+) or 1.6µg(++) of pCR3.1/Nef. Values represent the mean ± SD of triplicate determinations and are representative of two independent experiments. At least 100 individual cells were evaluated under each condition.
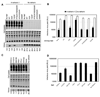
Figure 2. Effects of Nef substitution on HIV-1 particle release in the presence of human and monkey tetherin proteins
(A–H) Western blot analysis of HIV-1 particle release. A fixed amount (200ng) of each proviral plasmid was co-transfected with empty vector (none) or with increasing amounts (3.12ng, 6.25ng, or 12.5ng, left to right) of African green monkey (agm), human (hu), pig-tailed macaque (pgt), or rhesus macaque (rh-1) tetherin-HA expression plasmids. Cell and virion lysates were probed with an anti-capsid monoclonal antibody. Proviral plasmids carrying neither Nef nor Vpu (A) or HIV-1 Vpu (B) were used as controls. Thereafter, proviral plasmids lacking Vpu, but encoding HIV-1 Nef (C), SIVMAC Nef (D), SIVAGMSab Nef (E), SIVBLU Nef (F), SIVRCM Nef (G), or SIVGSN Nef (H) were used. Samples derived from each viral construct were run on a single gel, vertical lines are for visual guidance only. Left panels are film images following detection using chemiluminescence reagents, right panels show the results of p24CA quantitation in virion samples measured using quantitative fluorescence-based western blotting (LICOR). Results are from a single experiment and are representative of at least three separate experiments.
Figure 3. SIV Nef proteins expressed in trans can relieve inhibition of HIV-1 particle release by tetherins
(A–E) Western blot analysis of HIV-1 particle release. A fixed amount (200ng) of each proviral plasmid was co-transfected with a fixed amount (12.5ng) of rhesus macaque (rh-1), pig-tailed macaque (pgt), human (hu) or African green monkey (agm) tetherin-HA expression plasmids, along with varying amounts (0ng, 25ng, 50ng, 100ng) of HIV-1 Nef (A), SIVMAC Nef (B), SIVAGMSab Nef (C), SIVAGMTan Nef (D), or SIVBLU Nef (E) expression plasmids. Cell and virion lysates were probed with an anti-capsid monoclonal antibody. Samples derived using each Nef protein were run on a single gel, vertical lines are for visual guidance only. Left panels are film images following detection using chemiluminescence reagents, right panels show the results of p24CA quantitation in virion samples measured using quantitative fluorescence-based western blotting (LICOR). Results are from a single experiment and are representative of at least three separate experiments.
Figure 4. SIV Nef proteins can enhance virion release and relieve virion tethering in simian cells
(A) Rhesus macaque 221 cells and human 293T cells were infected with VSV-G pseudotyped SIVMAC239(WT) or SIVMAC239(del Nef), as indicated, at an MOI of 0.2 for 24h, then washed and incubated with 0, 10, 100 or 1000U/ml of IFNα for 24h. Cell and virion lysates were probed with anti-CA antibodies and signals revealed using fluorescence detection (LICOR, see Figure S6A, B for quantitation). (B) Rhesus macaque 221 cells were infected with VSV-G pseudotyped SIVMAC239(WT) (i and iii) or SIVMAC239(del Nef) (ii and iv), as indicated, at an MOI of 2 for 24h, then washed and incubated in the absence (i and ii) or presence (iii and iv) of 1000U/ml of IFNα for 24h. Cells were then fixed and examined using transmission electron microscopy. Scale bar = 500nm. (iv) inset, shows an expanded view of virions apparently tethered to each other. (C) African green monkey COS-7 cells were infected with VSV-G pseudotyped HIV-1 encoding no Nef, HIV-1 Nef or SIVAGMSab Nef, as indicated, at an MOI of 1 for 24h, then washed and incubated with 0, 10, 100 or 1000U/ml of IFNα for 24h. Cell and virion lysates were probed with anti-CA antibodies and signals revealed using fluorescence detection (LICOR, see Figure S6C for quantitation of virion release). Results are representative of at least three independent experiments.
Figure 5. The cytoplasmic tail of tetherin governs sensitivity to antagonism by SIVMAC Nef
(A) Schematic representation of the intact and chimeric tetherin proteins generated by exchange of sequences encoding the hu-tetherin and rh-tetherin cytoplasmic domains, generating huCT-rh-tetherin and rhCT-hu tetherin. (B) A fixed amount of (500ng) an HIV-1 proviral plasmid lacking both Vpu and Nef (upper panels) or lacking Vpu and encoding SIVMAC Nef (lower panels) was transfected either alone (− lanes) or along with 50ng of each intact or chimeric tetherin-HA expression plasmid depicted in (A) (+ lanes). The panels show western blots of cell and virion lysates probed with anti-capsid or anti-HA monoclonal antibodies, and detection with chemiluminescence based reagents. Numbers below each lane represent measurements of virion associated p24 measured using quantitative fluorescent western blotting (LICOR). (C) An HIV-1 proviral plasmid carrying SIVMAC Nef (500ng) was cotransfected with increasing amounts (0ng, 50ng, 100ng, 200ng) of each tetherin expression plasmid depicted in (A). Infectious virion yield was measured as in Figure 3. Results are the mean± SD of tripicate determinations from a single experiment and are representative of three independent experiments (D) Western blot analysis of SIVMAC239(WT) and SIVMAC239 (delNef) particle release in the presence of the intact and chimeric tetherin-HA proteins. Cells were transfected with a fixed amount (500ng) of each SIVMAC239 proviral plasmid and varying amounts (0, 50ng, 100ng) of each tetherin expression plasmid. The panels show western blots of cell and virion lysates probed with anti-capsid or anti-HA monoclonal antibodies, and detection with chemiluminescence based reagents. Numbers below each lane represent measurements of virion associated p27 measured using quantitative fluorescent western blotting (LICOR). (E) An SIVMAC239(WT) proviral plasmid was cotransfected with increasing amounts (0ng, 13ng, 25ng, 50ng, 100ng, 200ng) of each intact or chimeric tetherin expression plasmid depicted in (A). Infectious virion yield in the culture supernatant was measured as in Figure 3. Results are the mean± SD of tripicate determinations from a single experiment and are representative of three independent experiments
Figure 6. Insertion of a ‘missing’ five amino acid sequence in hu-tetherin confers sensitivity to Nef antagonism
(A) Alignment of the cytoplasmic tails of hu-tetherin and rh-tetherin-1 N-terminal cytoplasmic tails with differences in rh-tetherin-1 highlighted. Also shown are the three mutant hu-tetherin constructs that were used. (B) Western blot analysis of the expression of the mutant hu-tetherin proteins and inhibition of SIVMAC239(del Nef) particle release. Cells were transfected with a fixed amount (500ng) of proviral plasmid and 0, 12.5ng, 25ng, 50ng, 100ng or 200ng of each tetherin expression plasmid. Blots of cell lysates were simultaneously probed with rabbit anti-HA and (upper panels) and anti-CA monoclonal antibodies (middle panels). Lysates of pelleted virions were probed with anti-CA antibodies only. Signals were detected using fluorescent detection reagents (LICOR). (C) Same as (B), except that SIVMAC239(WT) was used in place of SIVMAC239(del Nef). (D) Quantitative analysis of virion associated p27CA, determined using the western blots shown in (B) for SIVMAC239(del Nef) and (C) for SIVMAC239(WT). Results are representative of at least three independent experiments.
Figure 7. Effects of introduced mutations and natural variation in the SIVMAC/SIVSMM/HIV-2 Nef proteins on rh-tetherin-1 antagonism
(A) Cells were transfected with 400ng of an HIV-1 proviral plasmid lacking Nef and Vpu, along with 25ng of the rh-tetherin expression plasmid and 100ng of each WT or mutant SIVMAC239 Nef expression plasmid. Blots of cell lysates were simultaneously probed with rabbit anti-HA and (upper panels) and anti-CA monoclonal antibodies (middle panels). Lysates of pelleted virions were probed with anti-CA antibodies only (lower panels). Signals were detected using quantitative fluorescent western blotting (LICOR) and numbers below each lane represent measurements of virion associated p24. (B) Cells were transfected as in (A) and the yield of infectious virions in the culture supernatant was measured using TZMbl indicator cells. Measurements are given in relative light units (RLU) following detection of β-galactosidase expression using a chemiluminescent assay. Results are the mean± SD of tripicate determinations from a single experiment and are representative of two independent experiments. (C) Cells were transfected with 400ng of an HIV-1 proviral plasmid lacking Nef and Vpu, along with 25ng of the rh-tetherin expression plasmid and 50ng of each HIV-2, SIVSMM or SIVMAC239 Nef expression plasmid. Blots were probed and analysed as in (A) and numbers below each lane represent measurements of virion associated p24. (D) Cells were transfected as in (C) and the yield of infectious virions in the culture supernatant was measured as in (B). Results are the mean± SD of tripicate determinations from a single experiment and are representative of two independent experiments.
Similar articles
- Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2.
Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Jia B, et al. PLoS Pathog. 2009 May;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436700 Free PMC article. - Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses.
Lim ES, Malik HS, Emerman M. Lim ES, et al. J Virol. 2010 Jul;84(14):7124-34. doi: 10.1128/JVI.00468-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444900 Free PMC article. - Counteraction of tetherin antiviral activity by two closely related SIVs differing by the presence of a Vpu gene.
Nikovics K, Dazza MC, Ekwalanga M, Mammano F, Clavel F, Saragosti S. Nikovics K, et al. PLoS One. 2012;7(4):e35411. doi: 10.1371/journal.pone.0035411. Epub 2012 Apr 17. PLoS One. 2012. PMID: 22530020 Free PMC article. - Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.
Tokarev A, Skasko M, Fitzpatrick K, Guatelli J. Tokarev A, et al. AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review. - BST-2/tetherin: a new component of the innate immune response to enveloped viruses.
Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. Evans DT, et al. Trends Microbiol. 2010 Sep;18(9):388-96. doi: 10.1016/j.tim.2010.06.010. Epub 2010 Aug 3. Trends Microbiol. 2010. PMID: 20688520 Free PMC article. Review.
Cited by
- Macaque-tropic human immunodeficiency virus type 1: breaking out of the host restriction factors.
Saito A, Akari H. Saito A, et al. Front Microbiol. 2013 Jul 9;4:187. doi: 10.3389/fmicb.2013.00187. eCollection 2013. Front Microbiol. 2013. PMID: 23847610 Free PMC article. - Breaking Barriers to an AIDS Model with Macaque-Tropic HIV-1 Derivatives.
Thippeshappa R, Ruan H, Kimata JT. Thippeshappa R, et al. Biology (Basel). 2012 May 12;1(2):134-64. doi: 10.3390/biology1020134. Biology (Basel). 2012. PMID: 23336082 Free PMC article. - Feline tetherin is characterized by a short N-terminal region and is counteracted by the feline immunodeficiency virus envelope glycoprotein.
Celestino M, Calistri A, Del Vecchio C, Salata C, Chiuppesi F, Pistello M, Borsetti A, Palù G, Parolin C. Celestino M, et al. J Virol. 2012 Jun;86(12):6688-700. doi: 10.1128/JVI.07037-11. Epub 2012 Apr 18. J Virol. 2012. PMID: 22514338 Free PMC article. - Differential Control of BST2 Restriction and Plasmacytoid Dendritic Cell Antiviral Response by Antagonists Encoded by HIV-1 Group M and O Strains.
Bego MG, Cong L, Mack K, Kirchhoff F, Cohen ÉA. Bego MG, et al. J Virol. 2016 Oct 28;90(22):10236-10246. doi: 10.1128/JVI.01131-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581991 Free PMC article. - Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu.
Goffinet C, Homann S, Ambiel I, Tibroni N, Rupp D, Keppler OT, Fackler OT. Goffinet C, et al. J Virol. 2010 Apr;84(8):4089-94. doi: 10.1128/JVI.01549-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147395 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01AI067057/AI/NIAID NIH HHS/United States
- R01 AI078788/AI/NIAID NIH HHS/United States
- R01 AI067057/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI050111/AI/NIAID NIH HHS/United States
- R01AI050111/AI/NIAID NIH HHS/United States
- R01AI078788/AI/NIAID NIH HHS/United States
- R01 AI073098/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous