Modulation of adaptive immunity with Toll-like receptors - PubMed (original) (raw)
Review
Modulation of adaptive immunity with Toll-like receptors
Santhakumar Manicassamy et al. Semin Immunol. 2009 Aug.
Abstract
The discovery of Toll-like receptors (TLRs), and their role in sensing infections represents one of the most seminal advances in immunology in recent years. It is now clear that TLRs play a fundamental role in innate recognition of microbes, and stimulate and tune the quality of the adaptive immune response. However, major knowledge gaps remain in our understanding of how TLRs regulate the development and persistence of T- and B-cell memory. Here, we review our current understanding of how TLR-signaling shapes the adaptive immune response, and highlight unanswered questions, the solution of which will be imperative in the rational exploitation of TLRs in vaccine design and immune therapy.
Figures
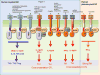
Figure 1. Modulation of adaptive immune responses by Toll-like receptors (TLRs) and its adaptor proteins
Activation of distinct TLRs in dendritic cells (DCs) results in the induction of distinct DC responses and adaptive immunities. In humans, TLRs 7 and 9 are expressed within the ER/phagolysosomes of pDCs, and signaling via these induces potent IFN-α, and induction of Th1 responses and cross-presentation of exogenous antigens to stimulate cytotoxic T cell (CTL) responses. In contrast, myeloid DCs in humans express TLR3 (in ER/phagolysosomal compartments), or TLR2 (heterodimerized with TLR1 or 6), or TLRs 3,4,5,8 and 11 on the surface. Stimulation via most TLRs induce potent IL-12p70 and Th1 responses. However, activation of TLR2 heterodimers (TLR2/1 or TLR2/6) produce relatively little IL-12 (p70) but robust IL-10, and skews the balance towards the Th0/Th2/T-regulatory phenotype. Interestingly, each TLR is associated with different adaptor proteins, which mediate distinct functions. For example, MyD88 signaling induces pro-inflammatory cytokines (IL-12, IL-6, and tumor necrosis factor) but not type I IFNs, and MyD88 does not upregulate costimulatory molecules. Signaling via TRIF or TRAM induces type I IFNs and upregulates costimulatory molecules.
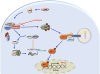
Figure 2. Regulation of TLR-mediated type I IFN production in plasmacytoid DCs, by mTOR
CpG or viral mediated activation of TLR9 leads to recruitment of MyD88 and TRAF6, and subsequent phosphorylation of IRF7, which promotes its translocation to the nucleus resulting in transcriptional activation of type I IFN genes. PI3K-AKT- signaling pathway activates mTOR which in-turn promotes TLR9-MyD88 complex formation through S6K phosphorylation and activation. Simultaneously, mTOR-mediated phosphorylation of 4E-BP results in dissociation of eukaryotic translation initiation factor (eIF)4E which binds to eIF4G/eIF4A complex and thus initiates more translation of IRF7 mRNA.
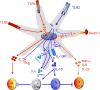
Figure 3
Programming DCs to induce Th1, Th2, Th17 or T regulatory responses. Signaling via TLR4 induces potent p38 and JNK1/2 MAPK activation which leads to the induction of interleukin-12 (IL-12) (p70). In contrast, Pam-3-cys, a TLR2/1 ligand, induce enhanced ERK 1/2 activation, which results in the stabilization of the transcription factor c-Fos that potently suppresses IL-12(p70) and enhances IL-10, thus favoring a Th2 bias. Interestingly, triggering DCs through TLR2/6 by zymosan efficiently induces ERK activation, which mediates induction of Raldh2. This results in the conversion of retinal to retinoic acid (RA), which then exerts an autocrine effect on DCs via RAR or RXR to induce SOSC3, which suppresses activation of p38 MAPK and proinflammatory cytokines. In contrast, zymosan mediated triggering of dectin-1in DCs promotes induction of proinflammatory cytokines IL-6 and IL-23 and thus mediates Th17 response.
Similar articles
- Regulation of B-cell responses by Toll-like receptors.
Browne EP. Browne EP. Immunology. 2012 Aug;136(4):370-9. doi: 10.1111/j.1365-2567.2012.03587.x. Immunology. 2012. PMID: 22444240 Free PMC article. Review. - Immunology: Toll-like receptors and antibody responses.
Nemazee D, Gavin A, Hoebe K, Beutler B. Nemazee D, et al. Nature. 2006 May 18;441(7091):E4; discussion E4. doi: 10.1038/nature04875. Nature. 2006. PMID: 16710369 Free PMC article. - Differential Effects of Toll-Like Receptor Signaling on the Activation of Immune Responses in the Upper Respiratory Tract.
Xu M, Li N, Fan X, Zhou Y, Bi S, Shen A, Wang B. Xu M, et al. Microbiol Spectr. 2022 Feb 23;10(1):e0114421. doi: 10.1128/spectrum.01144-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196817 Free PMC article. - Use of defined TLR ligands as adjuvants within human vaccines.
Duthie MS, Windish HP, Fox CB, Reed SG. Duthie MS, et al. Immunol Rev. 2011 Jan;239(1):178-96. doi: 10.1111/j.1600-065X.2010.00978.x. Immunol Rev. 2011. PMID: 21198672 Free PMC article. Review. - Toll-Like Receptors in Adaptive Immunity.
Kumar V. Kumar V. Handb Exp Pharmacol. 2022;276:95-131. doi: 10.1007/164_2021_543. Handb Exp Pharmacol. 2022. PMID: 34510306
Cited by
- Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency.
Kostinova AM, Latysheva EA, Kostinov MP, Akhmatova NK, Skhodova SA, Vlasenko AE, Cherdantsev AP, Soloveva IL, Khrapunova IA, Loktionova MN, Khromova EA, Poddubikov AA. Kostinova AM, et al. Vaccines (Basel). 2024 Jul 25;12(8):843. doi: 10.3390/vaccines12080843. Vaccines (Basel). 2024. PMID: 39203969 Free PMC article. - The Effect of Orally Administered Multi-Strain Probiotic Formulation (Lactobacillus, Bifidobacterium) on the Phagocytic Activity and Oxidative Metabolism of Peripheral Blood Granulocytes and Monocytes in Lambs.
Wójcik R, Małaczewska J, Tobolski D, Miciński J, Kaczorek-Łukowska E, Zwierzchowski G. Wójcik R, et al. Int J Mol Sci. 2024 May 7;25(10):5068. doi: 10.3390/ijms25105068. Int J Mol Sci. 2024. PMID: 38791112 Free PMC article. - Unraveling the skin; a comprehensive review of atopic dermatitis, current understanding, and approaches.
Afshari M, Kolackova M, Rosecka M, Čelakovská J, Krejsek J. Afshari M, et al. Front Immunol. 2024 Mar 4;15:1361005. doi: 10.3389/fimmu.2024.1361005. eCollection 2024. Front Immunol. 2024. PMID: 38500882 Free PMC article. Review. - SARS-CoV-2 spike-FLIPr fusion protein plus lipidated FLIPr protects against various SARS-CoV-2 variants in hamsters.
Hsieh M-S, Hsu C-W, Liao H-C, Lin C-L, Chiang C-Y, Chen M-Y, Liu S-J, Liao C-L, Chen H-W. Hsieh M-S, et al. J Virol. 2024 Feb 20;98(2):e0154623. doi: 10.1128/jvi.01546-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299865 Free PMC article. - OncoTherad® (MRB-CFI-1) Nanoimmunotherapy: A Promising Strategy to Treat Bacillus Calmette-Guérin-Unresponsive Non-Muscle-Invasive Bladder Cancer: Crosstalk among T-Cell CX3CR1, Immune Checkpoints, and the Toll-Like Receptor 4 Signaling Pathway.
Alonso JCC, de Souza BR, Reis IB, de Arruda Camargo GC, de Oliveira G, de Barros Frazão Salmazo MI, Gonçalves JM, de Castro Roston JR, Caria PHF, da Silva Santos A, de Freitas LLL, Billis A, Durán N, Fávaro WJ. Alonso JCC, et al. Int J Mol Sci. 2023 Dec 15;24(24):17535. doi: 10.3390/ijms242417535. Int J Mol Sci. 2023. PMID: 38139364 Free PMC article. Clinical Trial.
References
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. - PubMed
- Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. - PubMed
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. - PubMed
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. - PubMed
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI048638/AI/NIAID NIH HHS/United States
- R01 DK057665/DK/NIDDK NIH HHS/United States
- R01 AI048638/AI/NIAID NIH HHS/United States
- N01 AI050019/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- N01 AI050025/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources