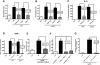Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways - PubMed (original) (raw)
Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways
Daniel Rittirsch et al. PLoS Pathog. 2009 Jun.
Abstract
Pathogen-pattern-recognition by Toll-like receptors (TLRs) and pathogen clearance after immune complex formation via engagement with Fc receptors (FcRs) represent central mechanisms that trigger the immune and inflammatory responses. In the present study, a linkage between TLR4 and FcgammaR was evaluated in vitro and in vivo. Most strikingly, in vitro activation of phagocytes by IgG immune complexes (IgGIC) resulted in an association of TLR4 with FcgammaRIII (CD16) based on co-immunoprecipitation analyses. Neutrophils and macrophages from TLR4 mutant (mut) mice were unresponsive to either lipopolysaccharide (LPS) or IgGIC in vitro, as determined by cytokine production. This phenomenon was accompanied by the inability to phosphorylate tyrosine residues within immunoreceptor tyrosine-based activation motifs (ITAMs) of the FcRgamma-subunit. To transfer these findings in vivo, two different models of acute lung injury (ALI) induced by intratracheal administration of either LPS or IgGIC were employed. As expected, LPS-induced ALI was abolished in TLR4 mut and TLR4(-/-) mice. Unexpectedly, TLR4 mut and TLR4(-/-) mice were also resistant to development of ALI following IgGIC deposition in the lungs. In conclusion, our findings suggest that TLR4 and FcgammaRIII pathways are structurally and functionally connected at the receptor level and that TLR4 is indispensable for FcgammaRIII signaling via FcRgamma-subunit activation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
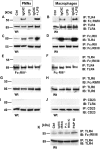
Figure 1. Association between TLR4 and FcRγIII.
Peritoneal PMNs and macrophages (3×106 cells/ml) from Wt mice and FcRγ-subunit−/− mice were incubated in vitro for 30 min with either IgG immune complexes (IgGIC; 100 µg/ml), LPS (20 ng/ml), or the combination. (A,B) Western blot analysis (IB) for FcγRIII of Wt PMN or macrophage lysates co-immunoprecipitated (IP) with anti-TLR4. (C,D) Reverse direction immunoprecipitation using anti-FcγRII/III IgG followed by Western blot analysis for TLR4. (E,F) Western blot analysis for FcγRIII of PMNs or macrophages from FcγRIII−/− co-immunoprecipitated (IP) with anti-TLR4. (G,H) Samples were immunoprecipitated with anti-TLR6 IgG and probed for FcγRIII. (I,J) Immunoprecipitation with anti-CD23 followed by Western blots using anti-TLR4 IgG. (K) Western blots (IB) of cell lysates of Wt macrophages that were incubated for 30 min with BSA IgG immune complexes (IgGIC; 100 µg/ml), polymyxin-treated BSA IgG immune complexes (p.-t. BSA IC; 100 µg/ml) or peroxidase/anti-peroxidase IgGIC immune complexes (PAP IC, 100 µg/ml). IB for FcγRIII of Wt macrophage lysates co-immunoprecipitated (IP) with anti-TLR4. Corresponding loading controls are displayed in lower panels.
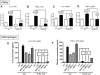
Figure 2. In vitro cytokine responses of elicited peritoneal PMNs and macrophages to LPS and IgGIC.
In vitro cytokine responses of elicited peritoneal PMNs (A–D) and macrophages (E,F). Cells (3×106 cells/ml) from either Wt or TLR4 mut mice were incubated for 4 hr with LPS (20 ng/ml) or IgGIC; 100 µg/ml), respectively. In addition, macrophages were incubated with polymyxin-treated BSA IgG immune complexes (p.-t. BSA IC, 100 µg/ml) or peroxidase/anti-peroxidase IgGIC immune complexes (PAP IC, 100 µg/ml). (A) IL-6 release from PMNs after LPS stimulation. (B) TNFα levels after incubation of PMNs with LPS. (C) Concentration of IL-6 in supernatants when PMNs were exposed to IgGIC. (D) Production of TNFα by PMNs and macrophages in the presence of IgGIC. Ctrl = control levels of non-stimulated cells. (E) Release of IL-6 by macrophages into supernatant fluids after stimulation with LPS, IgGIC, p.-t. BSA IC, or PAP IC. (F) TNFα production by macrophages exposed to LPS, IgGIC, p.-t. BSA IC, or PAP IC. The experiments were performed in triplicates for each condition (each bar) with n≥3 donors of cells for each mouse strain, Wt or TLR4 mut. Differences between controls and stimulated cells were—if not otherwise noted—statistically significant (p<0.05).
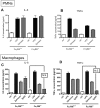
Figure 3. Responsiveness of FcγRIII-deficient phagocytes to LPS.
Peritoneal PMNs (A,B) and macrophages (C,D) from Wt and FcγRIII−/− mice were incubated to LPS (100 ng/ml) or Zymosan (300 µg/ml), or IgG immune complexes (IgGIC; 100 µg/ml; macrophages only), and supernatant fluids were analyzed for IL-6 and TNFα levels. Ctrl = control levels of non-stimulated cells. For each condition, n≥4. Differences between controls and stimulated cells were—if not otherwise noted—statistically significant (p<0.05).
Figure 4. Western blot analysis for tyrosine-phosphorylated (PY) FcRγ-subunit of PMN or macrophage lysates after in vitro incubation.
(A,B) 3×106 cells/ml from either Wt or TLR4 mut mice were incubated for 5, 15, and 30 min with IgG immune complexes (IgGIC; 100 µg/ml). (C,D) The same protocol was used for stimulation with LPS (20 ng/ml). (E) Lysates from either Wt or TLR4 mut mice that were incubated with polymyxin-treated BSA immune complexes (100 µg/ml) under the same conditions as described above. Corresponding loading controls are displayed in the lower panels.
Figure 5. Parameters of acute lung injury in Wt and TLR4 mut mice.
(A) Lung injury (as measured by leak of 125I-BSA into lung) in Wt, TLR4 mut, TLR4+/+, and TLR4−/− mice receiving LPS intratracheally. (B) Permeability indices in Wt, TLR4 mut, TLR4+/+, and TLR4−/− mice after intrapulmonary immune complex formation following administration of BSA (i.v.) and anti-BSA IgG (i.t.). (C) IL-6 levels in BAL fluids after IgG immune complex (IgGIC)- or LPS-induced lung injury using Wt and TLR4 mut mice. (D) TNFα in BAL fluids from the same mice described in frame (C). For each bar, n≥5. (E) Lung injury induced by IgG immune complexes (IgGIC) in Wt and TLR4 mut mice after endotoxin removal by polymyxin. (F) Lung permeability after intratracheal (i.t.) administration of anti-BSA IgG and intravenous (i.v.) injection of BSA, PBS i.t., and BSA i.v. or anti-BSA i.t. and PBS i.v. (G) IgGIC-induced lung injury in FcRγ-subunit−/− mice in comparison to Wt mice (FcRγ-subunit+/+). For each bar, n≥5.
Figure 6. Expression levels of FcγRII/III, FcRγ-subunit, and C5aR on phagocytes from Wt and TLR4 mut mice.
(A) Summary of flow cytometry analyses of FcγRII/III expression on blood PMNs. (B,C) Original flow cytometry results for FcγRII/III expression on the surface of PMNs from Wt (B) or TLR4 mut (C) mice. (D,E) Analysis of the expression of FcγRIII (upper bands) and FcRγ-subunit (middle bands) in cell lysates [(D), PMNs; (E), macrophages] from Wt or TLR4 mut mice by Western blotting. The lower bands represent the analysis for GAPDH as loading controls. (F) Surface expression of C5aR protein on PMNs from Wt or TLR4 mut mice as assessed by flow cytometry. MFI, mean fluorescence intensity. Studies were done in three separate and independent experiments, with each sample run in duplicates.
Similar articles
- Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation.
Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nürnberg B, Spicher K, Birnbaumer L, Zwirner J, Claassens JW, Verbeek JS, van Rooijen N, Köhl J, Gessner JE. Skokowa J, et al. J Immunol. 2005 Mar 1;174(5):3041-50. doi: 10.4049/jimmunol.174.5.3041. J Immunol. 2005. PMID: 15728518 - THP-1 cells increase TNF-α production upon LPS + soluble human IgG co-stimulation supporting evidence for TLR4 and Fcγ receptors crosstalk.
Vargas-Hernández O, Ventura-Gallegos JL, Ventura-Ayala ML, Torres M, Zentella A, Pedraza-Sánchez S. Vargas-Hernández O, et al. Cell Immunol. 2020 Sep;355:104146. doi: 10.1016/j.cellimm.2020.104146. Epub 2020 Jun 12. Cell Immunol. 2020. PMID: 32702524 - Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a.
Syed SN, Konrad S, Wiege K, Nieswandt B, Nimmerjahn F, Schmidt RE, Gessner JE. Syed SN, et al. Eur J Immunol. 2009 Dec;39(12):3343-56. doi: 10.1002/eji.200939884. Eur J Immunol. 2009. PMID: 19795417 - The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases.
Karsten CM, Köhl J. Karsten CM, et al. Immunobiology. 2012 Nov;217(11):1067-79. doi: 10.1016/j.imbio.2012.07.015. Immunobiology. 2012. PMID: 22964232 Review. - On the role of complement and Fc gamma-receptors in the Arthus reaction.
Köhl J, Gessner JE. Köhl J, et al. Mol Immunol. 1999 Sep-Oct;36(13-14):893-903. doi: 10.1016/s0161-5890(99)00111-x. Mol Immunol. 1999. PMID: 10698344 Review.
Cited by
- Presentation of phagocytosed antigens by MHC class I and II.
Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Mantegazza AR, et al. Traffic. 2013 Feb;14(2):135-52. doi: 10.1111/tra.12026. Epub 2012 Nov 29. Traffic. 2013. PMID: 23127154 Free PMC article. Review. - Microbial manipulation of receptor crosstalk in innate immunity.
Hajishengallis G, Lambris JD. Hajishengallis G, et al. Nat Rev Immunol. 2011 Mar;11(3):187-200. doi: 10.1038/nri2918. Nat Rev Immunol. 2011. PMID: 21350579 Free PMC article. Review. - Selective antibody intervention of Toll-like receptor 4 activation through Fc γ receptor tethering.
Shang L, Daubeuf B, Triantafilou M, Olden R, Dépis F, Raby AC, Herren S, Dos Santos A, Malinge P, Dunn-Siegrist I, Benmkaddem S, Geinoz A, Magistrelli G, Rousseau F, Buatois V, Salgado-Pires S, Reith W, Monteiro R, Pugin J, Leger O, Ferlin W, Kosco-Vilbois M, Triantafilou K, Elson G. Shang L, et al. J Biol Chem. 2014 May 30;289(22):15309-18. doi: 10.1074/jbc.M113.537936. Epub 2014 Apr 15. J Biol Chem. 2014. PMID: 24737331 Free PMC article. - Acute Kidney Injury Induced Lupus Exacerbation Through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice With Renal Ischemia Reperfusion Injury.
Saisorn W, Saithong S, Phuengmaung P, Udompornpitak K, Bhunyakarnjanarat T, Visitchanakun P, Chareonsappakit A, Pisitkun P, Chiewchengchol D, Leelahavanichkul A. Saisorn W, et al. Front Immunol. 2021 Jun 24;12:669162. doi: 10.3389/fimmu.2021.669162. eCollection 2021. Front Immunol. 2021. PMID: 34248948 Free PMC article. - Prolyl Oligopeptidase From Leishmania infantum: Biochemical Characterization and Involvement in Macrophage Infection.
Lasse C, Azevedo CS, de Araújo CN, Motta FN, Andrade MA, Rocha AP, Sampaio I, Charneau S, Gèze M, Grellier P, Santana JM, Bastos IMD. Lasse C, et al. Front Microbiol. 2020 May 28;11:1060. doi: 10.3389/fmicb.2020.01060. eCollection 2020. Front Microbiol. 2020. PMID: 32547514 Free PMC article.
References
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. - PubMed
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. - PubMed
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
- Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL031963/HL/NHLBI NIH HHS/United States
- R37 GM029507/GM/NIGMS NIH HHS/United States
- R01 GM061656/GM/NIGMS NIH HHS/United States
- GM-29507/GM/NIGMS NIH HHS/United States
- R01 GM029507/GM/NIGMS NIH HHS/United States
- GM-61656/GM/NIGMS NIH HHS/United States
- HL-31963/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials