Leber congenital amaurosis caused by Lebercilin (LCA5) mutation: retained photoreceptors adjacent to retinal disorganization - PubMed (original) (raw)
. 2009 Jun 2:15:1098-106.
Affiliations
- PMID: 19503738
- PMCID: PMC2690955
Leber congenital amaurosis caused by Lebercilin (LCA5) mutation: retained photoreceptors adjacent to retinal disorganization
Samuel G Jacobson et al. Mol Vis. 2009.
Abstract
Purpose: To determine the retinal disease expression in the rare form of Leber congenital amaurosis (LCA) caused by Lebercilin (LCA5) mutation.
Methods: Two young unrelated LCA patients, ages six years (P1) and 25 years (P2) at last visit, both with the same homozygous mutation in the LCA5 gene, were evaluated clinically and with noninvasive studies. En face imaging was performed with near-infrared (NIR) reflectance and autofluorescence (AF); cross-sectional retinal images were obtained with optical coherence tomography (OCT). Dark-adapted thresholds were measured in the older patient; and the transient pupillary light reflex was recorded and quantified in both patients.
Results: Both LCA5 patients had light perception vision only, hyperopia, and nystagmus. P1 showed a prominent central island of retinal pigment epithelium (RPE) surrounded by alternating elliptical-appearing areas of decreased and increased pigmentation. Retinal laminar architecture at and near the fovea was abnormal in both patients. Foveal outer nuclear layer (ONL) was present in P1 and P2 but to different degrees. With increasing eccentricity, there was retinal laminar disorganization. Regions of pericentral and midperipheral retina in P1, but not P2, could retain measurable ONL and less laminopathy. P2 had a small central island of perception with >5 log units of sensitivity loss. Pupillary responsiveness was present in both LCA5 patients; the thresholds were abnormally elevated by >or=5.5 log units.
Conclusions: LCA5 patients had evidence of retained photoreceptors mainly in the central retina. Retinal remodeling was present in pericentral regions in both patients. The NIR reflectance and NIR-AF imaging in the younger patient suggested preserved RPE in retinal regions with retained photoreceptors. Detailed phenotype studies in other LCA5 patients with longitudinal follow-up will help determine the feasibility of future intervention in this rare disease.
Figures
Figure 1
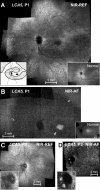
En face near-infrared reflectance and autofluorescence images of the LCA5 patients. A: Near-infrared (NIR) reflectance (REF) image of the left fundus of P1 is shown. Inset to the left is a schematic drawing of the retinal regions corresponding to low reflection (black), intermediate reflection (hatched), and high reflection and choroidal visibility (white). Inset to the right is a NIR reflectance view of the fundus of a 6 year-old child with normal vision. B: Near-infrared-autofluorescence (NIR-AF) image of the left fundus of P1 is shown. Black arrows indicate the boundaries of the midperipheral transitions to healthier retinal pigment epithelium; and gray arrow points to the parafoveal annular region of low intensity. Inset is a normal image. C: NIR reflectance image of the left fundus of P2 is shown. Inset is an enlarged view of the optic nerve head (ONH) region with ONH drusen. D: NIR-AF image of the left fundus of P2 is shown. Inset is an enlarged view of the ONH region. All images are shown contrast stretched for visibility of features.
Figure 2
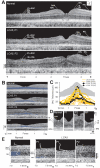
Dysmorphology in the retina of LCA5. A: Cross-sectional OCT images across the horizontal meridian are shown for a normal 6-year-old subject (upper) and LCA5, P1 (middle) and P2 (lower). Layers or structures are labeled as individual or combined laminae. IPL+RGC, inner plexiform and retinal ganglion cell layers; INL, inner nuclear layer; ONL, outer nuclear layer; OLM, outer limiting membrane; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium. N: Nasal, T: Temporal retina. B: Central scans are shown for a normal subject, P1, P2, and an RP patient with a residual and abnormally reduced central island of retinal structure. ONL layer is highlighted in blue. C: Photoreceptor nuclear layer thickness horizontally across the central 4 mm of retina is graphically displayed for a group of normal subjects (gray represents mean±2SD; n=26; ages 5–58 years), 19 patients with retinal degeneration but not LCA5, and the LCA5 data. For comparison with LCA5 P1 and P2 with light perception (LP) vision, the ONL data from the patients with retinal degeneration are color-coded by their visual acuity levels. D: Magnified (1.2 mm across) horizontal cross-sections through the fovea of LCA5 P1 and P2 are compared to those of the 6-year-old normal subject (left panel) and an RP patient (right panel) with similarly reduced foveal ONL. E: Cross-sectional, 0.9 mm-long, extramacular images from LCA5 P1 and P2 are compared to a normal subject. Longitudinal reflectivity profiles (LRP, white traces) overlaid on the scans show signal features corresponding to the different retinal laminae. The ONL is highlighted (blue) next to the corresponding LRP signal feature. LCA5 P1 (left) at 7 to 7.8 mm in nasal retina shows remnants of ONL, retained retinal lamination and a thickened inner retina (bracketed to the left of the scans) compared to the normal subject at the same eccentricity. Scans from 7 mm in temporal retina from both LCA5 patients show complete loss of ONL signal and retinal disorganization with a bilaminar appearance of the LRPs.
Figure 3
Transient pupillary light reflex (TPLR) abnormalities in LCA5 patients. A: Change in pupil diameter is plotted as a function of time in response to short duration (0.1 s) light stimuli of two different intensities (0.4 and 1.4 log scot-cd.s.m−2; denoted at right end of traces) in the LCA5 patients (filled symbols). A response elicited with the brighter stimulus in a 9-year-old normal subject is shown for comparison (unfilled symbols). Stimulus monitor is shown at lower left of each of the two panels. B: TPLR response thresholds to a 0.3 mm criterion response in the LCA5 patients (filled symbols) are compared to a group of 11 LCA patients, age 1–58 years, with severely impaired vision (unfilled symbols). TPLR response amplitudes are measured at a fixed time of 0.9s (vertical dashed line in A). Thresholds in the LCA5 patients and other LCA patients show elevations in excess of 5 log units from normal. Gray hexagon denotes normal mean±2SD.
Similar articles
- Treatment Potential for LCA5-Associated Leber Congenital Amaurosis.
Uyhazi KE, Aravand P, Bell BA, Wei Z, Leo L, Serrano LW, Pearson DJ, Shpylchak I, Pham J, Vasireddy V, Bennett J, Aleman TS. Uyhazi KE, et al. Invest Ophthalmol Vis Sci. 2020 May 11;61(5):30. doi: 10.1167/iovs.61.5.30. Invest Ophthalmol Vis Sci. 2020. PMID: 32428231 Free PMC article. - Screening of a large cohort of leber congenital amaurosis and retinitis pigmentosa patients identifies novel LCA5 mutations and new genotype-phenotype correlations.
Mackay DS, Borman AD, Sui R, van den Born LI, Berson EL, Ocaka LA, Davidson AE, Heckenlively JR, Branham K, Ren H, Lopez I, Maria M, Azam M, Henkes A, Blokland E, Qamar R, Webster AR, Cremers FPM, Moore AT, Koenekoop RK; [LCA5 Study Group (see acknowledgements for Universities); Andreasson S, de Baere E, Bennett J, Chader GJ, Berger W, Golovleva I, Greenberg J, den Hollander AI, Klaver CCW, Klevering BJ, Lorenz B, Preising MN, Ramsear R, Roberts L, Roepman R, Rohrschneider K, Wissinger B. Mackay DS, et al. Hum Mutat. 2013 Nov;34(11):1537-1546. doi: 10.1002/humu.22398. Epub 2013 Sep 17. Hum Mutat. 2013. PMID: 23946133 Free PMC article. - Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations.
Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Windsor EA, Schwartz SB, Heon E, Stone EM. Jacobson SG, et al. Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4573-7. doi: 10.1167/iovs.08-2121. Epub 2008 Jun 6. Invest Ophthalmol Vis Sci. 2008. PMID: 18539930 Free PMC article. - Leber congenital amaurosis: genes, proteins and disease mechanisms.
den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. den Hollander AI, et al. Prog Retin Eye Res. 2008 Jul;27(4):391-419. doi: 10.1016/j.preteyeres.2008.05.003. Epub 2008 Jun 1. Prog Retin Eye Res. 2008. PMID: 18632300 Review. - Measures of Function and Structure to Determine Phenotypic Features, Natural History, and Treatment Outcomes in Inherited Retinal Diseases.
Cideciyan AV, Krishnan AK, Roman AJ, Sumaroka A, Swider M, Jacobson SG. Cideciyan AV, et al. Annu Rev Vis Sci. 2021 Sep 15;7:747-772. doi: 10.1146/annurev-vision-032321-091738. Epub 2021 Jul 13. Annu Rev Vis Sci. 2021. PMID: 34255540 Review.
Cited by
- Full-Field Pupillary Light Responses, Luminance Thresholds, and Light Discomfort Thresholds in CEP290 Leber Congenital Amaurosis Patients.
Collison FT, Park JC, Fishman GA, McAnany JJ, Stone EM. Collison FT, et al. Invest Ophthalmol Vis Sci. 2015 Nov;56(12):7130-6. doi: 10.1167/iovs.15-17467. Invest Ophthalmol Vis Sci. 2015. PMID: 26529047 Free PMC article. - Treatment Potential for LCA5-Associated Leber Congenital Amaurosis.
Uyhazi KE, Aravand P, Bell BA, Wei Z, Leo L, Serrano LW, Pearson DJ, Shpylchak I, Pham J, Vasireddy V, Bennett J, Aleman TS. Uyhazi KE, et al. Invest Ophthalmol Vis Sci. 2020 May 11;61(5):30. doi: 10.1167/iovs.61.5.30. Invest Ophthalmol Vis Sci. 2020. PMID: 32428231 Free PMC article. - Leber's Congenital Amaurosis: Current Concepts of Genotype-Phenotype Correlations.
Huang CH, Yang CM, Yang CH, Hou YC, Chen TC. Huang CH, et al. Genes (Basel). 2021 Aug 19;12(8):1261. doi: 10.3390/genes12081261. Genes (Basel). 2021. PMID: 34440435 Free PMC article. Review. - Human retina-in-a-dish: Unlocking the potential to study mechanisms of inherited retinal disease.
Yee T, Wert KJ. Yee T, et al. Mol Ther Methods Clin Dev. 2023 Sep 3;30:573-575. doi: 10.1016/j.omtm.2023.08.019. eCollection 2023 Sep 14. Mol Ther Methods Clin Dev. 2023. PMID: 37693947 Free PMC article. No abstract available. - Identification of a novel LCA5 mutation in a Pakistani family with Leber congenital amaurosis and cataracts.
Ahmad A, Daud S, Kakar N, Nürnberg G, Nürnberg P, Babar ME, Thoenes M, Kubisch C, Ahmad J, Bolz HJ. Ahmad A, et al. Mol Vis. 2011;17:1940-5. Epub 2011 Jul 16. Mol Vis. 2011. PMID: 21850168 Free PMC article.
References
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. - PubMed
- Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144:791–811. - PubMed
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–82. - PMC - PubMed
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, Windsor EA, Sumaroka A, Pearce-Kelling SE, Conlon TJ, Chiodo VA, Boye SL, Flotte TR, Maguire AM, Bennett J, Hauswirth WW. Safety of recombinant adeno-associated virus type 2–RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13:1074–84. - PubMed
- Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, Cheung AY, Sumaroka A, Pearce-Kelling SE, Aguirre GD, Kaushal S, Maguire AM, Flotte TR, Hauswirth WW. Safety in nonhuman primates of ocular AAV2–RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006;17:845–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous