Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1186/1475-2875-8-125.
Milijaona Randrianarivelojosia, Issaka Sagara, Philippe Brasseur, Ibrahima Ndiaye, Babacar Faye, Laurence Randrianasolo, Arsène Ratsimbasoa, Doris Forlemu, Vicky Ama Moor, Aminata Traore, Yahia Dicko, Niawanlou Dara, Valérie Lameyre, Mouctar Diallo, Abdoulaye Djimde, Albert Same-Ekobo, Oumar Gaye
Affiliations
- PMID: 19505304
- PMCID: PMC2698916
- DOI: 10.1186/1475-2875-8-125
Randomized Controlled Trial
Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria
Jean Louis Ndiaye et al. Malar J. 2009.
Abstract
Background: The use of artemisinin derivative-based combination therapy (ACT) such as artesunate plus amodiaquine is currently recommended for the treatment of uncomplicated Plasmodium falciparum malaria. Fixed-dose combinations are more adapted to patients than regimens involving multiple tablets and improve treatment compliance. A fixed-dose combination of artesunate + amodiaquine (ASAQ) was recently developed. To assess the efficacy and safety of this new combination and to define its optimum dosage regimen (once or twice daily) in the treatment of uncomplicated P. falciparum malaria, a multicentre clinical study was conducted.
Methods: A multicentre, randomized, controlled, investigator-blinded, parallel-group study was conducted in five African centers in Cameroon, Madagascar, Mali and Senegal from March to December 2006. Efficacy and safety of ASAQ were assessed compared to those of artemether + lumefantrine (AL). The WHO protocol with a 28-day follow-up for assessing the drug therapeutic efficacy was used. Patients suffering from uncomplicated P. falciparum malaria were randomized to receive ASAQ orally once daily (ASAQ1), ASAQ twice daily (ASAQ2) or AL twice daily (AL) for three days. The primary outcome was PCR-corrected parasitological cure rate and clinical response.
Results: Of 941 patients initially randomized and stratified into two age groups (<5 years, and >or=5 years), 936 (99.5%) were retained for the intent to treat (ITT) analysis, and 859 (91.3%) patients for the per protocol (PP) analysis. Among ITT population, up to D28, PCR-corrected adequate parasitological and clinical response rates were 95.2% in the ASAQ1 group, 94.9% in the ASAQ2 group and 95.5% in the AL group. Moreover, the cure rate evaluated among PP population was >or=98.5% in both ASAQ therapeutic arms. Therapeutic response rates did not display any significant differences between age groups or between one geographical site and another. Altogether, this demonstrates the non-inferiority of ASAQ1 regimen compared to both ASAQ2 and AL regimens. During follow-up mild and moderate adverse events including gastrointestinal and/or nervous disorders were reported in 29.3% of patients, with no difference between groups in the nature, frequency or intensity of adverse events.
Conclusion: The non-inferiority of ASAQ compared with AL was demonstrated. The fixed-dose combination artesunate + amodiaquine (ASAQ) is safe and efficacious even in young children under 5 years of age. Whilst administration on a twice-a-day basis does not improve the efficacy of ASAQ significantly, a once-a-day intake of this new combination clearly appears as an effective and safe therapy in the treatment of uncomplicated P. falciparum malaria both in adults and children. Implications of such findings are of primary importance in terms of public health especially in African countries. As most national policies plan to strengthen malaria control to reach the elimination of this disease, anti-malarial drugs such as the artesunate + amodiaquine fixed-dose ACT will play a pivotal role in this process.
Trial registration: The protocol was registered with the www.clinicaltrials.gov open clinical trial registry under the identifier number NCT00316329.
Figures
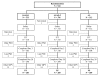
Figure 1
Patient flow through the study. AS: artesunate; AQ: amodiaquine; A: artemether; L: lumefantrine; ITT: intent to treat; PP: per protocol; MPV: major protocol violation; PSD: premature study discontinuation.
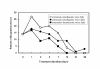
Figure 2
Gametocyte clearance. Data are presented as the number of patients with gametocytes.
Similar articles
- Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial.
Ndiaye JL, Faye B, Gueye A, Tine R, Ndiaye D, Tchania C, Ndiaye I, Barry A, Cissé B, Lameyre V, Gaye O. Ndiaye JL, et al. Malar J. 2011 Aug 12;10:237. doi: 10.1186/1475-2875-10-237. Malar J. 2011. PMID: 21838909 Free PMC article. Clinical Trial. - In vivo/ex vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: an open label randomized controlled trial in Burkina Faso.
Lingani M, Bonkian LN, Yerbanga I, Kazienga A, Valéa I, Sorgho H, Ouédraogo JB, Mens PF, Schallig HDFH, Ravinetto R, d'Alessandro U, Tinto H. Lingani M, et al. Malar J. 2020 Jan 6;19(1):8. doi: 10.1186/s12936-019-3089-z. Malar J. 2020. PMID: 31906948 Free PMC article. Clinical Trial. - Artesunate-amodiaquine fixed dose combination for the treatment of Plasmodium falciparum malaria in India.
Anvikar AR, Sharma B, Shahi BH, Tyagi PK, Bose TK, Sharma SK, Srivastava P, Srivastava B, Kiechel JR, Dash AP, Valecha N. Anvikar AR, et al. Malar J. 2012 Mar 30;11:97. doi: 10.1186/1475-2875-11-97. Malar J. 2012. PMID: 22458860 Free PMC article. Clinical Trial. - Monitoring the Efficacy and Safety of Artemisinin-Based Combination Therapies: A Review and Network Meta-analysis of Antimalarial Therapeutic Efficacy Trials in Cameroon.
Whegang Youdom S, Chiabi A, Basco LK. Whegang Youdom S, et al. Drugs R D. 2019 Mar;19(1):1-14. doi: 10.1007/s40268-018-0259-3. Drugs R D. 2019. PMID: 30656608 Free PMC article. Review. - Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria.
Bukirwa H, Unnikrishnan B, Kramer CV, Sinclair D, Nair S, Tharyan P. Bukirwa H, et al. Cochrane Database Syst Rev. 2014 Mar 4;(3):CD006404. doi: 10.1002/14651858.CD006404.pub2. Cochrane Database Syst Rev. 2014. PMID: 24596021 Free PMC article. Updated. Review.
Cited by
- Slow clearance of histidine-rich protein-2 in Gabonese with uncomplicated malaria.
Lamsfus Calle C, Schaumburg F, Rieck T, Nkoma Mouima AM, Martinez de Salazar P, Breil S, Behringer J, Kremsner PG, Mordmüller B, Fendel R. Lamsfus Calle C, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0099424. doi: 10.1128/spectrum.00994-24. Epub 2024 Aug 28. Microbiol Spectr. 2024. PMID: 39194289 Free PMC article. - Therapeutic efficacy and safety of artesunate + amodiaquine and artemether + lumefantrine in treating uncomplicated Plasmodium falciparum malaria in children on the rainy south-east coast of Madagascar.
Irinantenaina J, Carn G, Randriamiarinjatovo DNAL, Harimanana AN, Razanatsiorimalala S, Ralemary N, Randriarison M, Razafinjato C, Hotahiene R, Randrianarivelojosia M. Irinantenaina J, et al. Parasite. 2023;30:32. doi: 10.1051/parasite/2023034. Epub 2023 Aug 30. Parasite. 2023. PMID: 37646608 Free PMC article. Clinical Trial. - Effectiveness and safety of artesunate-amodiaquine versus artemether-lumefantrine for home-based treatment of uncomplicated Plasmodium falciparum malaria among children 6-120 months in Yaoundé, Cameroon: a randomized trial.
Niba PTN, Nji AM, Ali IM, Akam LF, Dongmo CH, Chedjou JPK, Fomboh CT, Nana WD, Oben OLA, Selly-Ngaloumo AA, Moyeh MN, Ngu JA, Ludovic AJ, Aboh PM, Ambani MCE, Omgba PAM, Kotcholi GB, Adzemye LM, Nna DRA, Douanla A, Ango Z, Ewane MS, Ticha JT, Tatah FM, Dinza G, Ndikum VN, Fosah DA, Bigoga JD, Alifrangis M, Mbacham WF. Niba PTN, et al. BMC Infect Dis. 2022 Feb 21;22(1):166. doi: 10.1186/s12879-022-07101-2. BMC Infect Dis. 2022. PMID: 35189818 Free PMC article. Clinical Trial. - Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Mali: a systematic review and meta-analysis.
Maiga FO, Wele M, Toure SM, Keita M, Tangara CO, Refeld RR, Thiero O, Kayentao K, Diakite M, Dara A, Li J, Toure M, Sagara I, Djimdé A, Mather FJ, Doumbia SO, Shaffer JG. Maiga FO, et al. Malar J. 2021 Aug 30;20(1):356. doi: 10.1186/s12936-021-03890-0. Malar J. 2021. PMID: 34461901 Free PMC article. - Drug resistance markers within an evolving efficacy of anti-malarial drugs in Cameroon: a systematic review and meta-analysis (1998-2020).
Niba PTN, Nji AM, Evehe MS, Ali IM, Netongo PM, Ngwafor R, Moyeh MN, Ngum LN, Ndum OE, Acho FA, Mbu'u CM, Fosah DA, Atogho-Tiedeu B, Achonduh-Atijegbe O, Djokam-Dadjeu R, Chedjou JPK, Bigoga JD, Moukoko CEE, Ajua A, Achidi E, Tallah E, Leke RGF, Tourgordi A, Ringwald P, Alifrangis M, Mbacham WF. Niba PTN, et al. Malar J. 2021 Jan 9;20(1):32. doi: 10.1186/s12936-020-03543-8. Malar J. 2021. PMID: 33422080 Free PMC article.
References
- World Health Organisation . Antimalarial drug combination therapy: report of a technical consultation. Geneva: WHO; 2001.
- Adjuik M, Agnamey P, Babiker A, Borrmann S, Brasseur P, Cisse M, Cobelens F, Diallo S, Faucher JF, Garner P, Gikunda S, Kremsner PG, Krishna S, Lell B, Loolpapit M, Matsiegui PB, Missinou MA, Mwanza J, Ntoumi F, Olliaro P, Osimbo P, Rezbach P, Some E, Taylor WR. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet. 2002;359:1365–1372. doi: 10.1016/S0140-6736(02)08348-4. - DOI - PubMed
- Ndiaye JL, Faye B, Diouf AM, Kuete T, Cisse M, Seck PA, Brasseur P, Same-Ekobo A, Lameyre V, Gaye O. Randomized, comparative study of the efficacy and safety of artesunate plus amodiaquine, administered as a single daily intake versus two daily intakes in the treatment of uncomplicated falciparum malaria. Malar J. 2008;7:16. doi: 10.1186/1475-2875-7-16. - DOI - PMC - PubMed
- Tall A, Rabarijaona LP, Robert V, Bedja SA, Ariey F, Randrianarivelojosia M. Efficacy of artesunate plus amodiaquine, artesunate plus sulfadoxine-pyrimethamine, and chloroquine plus sulfadoxine-pyrimethamine in patients with uncomplicated Plasmodium falciparum in the Comoros Union. Acta Trop. 2007;102:176–181. doi: 10.1016/j.actatropica.2007.03.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous