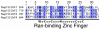Crystallographic and biochemical analysis of the Ran-binding zinc finger domain - PubMed (original) (raw)
Crystallographic and biochemical analysis of the Ran-binding zinc finger domain
James R Partridge et al. J Mol Biol. 2009.
Abstract
The nuclear pore complex (NPC) resides in circular openings within the nuclear envelope and serves as the sole conduit to facilitate nucleocytoplasmic transport in eukaryotes. The asymmetric distribution of the small G protein Ran across the nuclear envelope regulates directionality of protein transport. Ran interacts with the NPC of metazoa via two asymmetrically localized components, Nup153 at the nuclear face and Nup358 at the cytoplasmic face. Both nucleoporins contain a stretch of distinct, Ran-binding zinc finger domains. Here, we present six crystal structures of Nup153-zinc fingers in complex with Ran and a 1.48 A crystal structure of RanGDP. Crystal engineering allowed us to obtain well diffracting crystals so that all ZnF-Ran complex structures are refined to high resolution. Each of the four zinc finger modules of Nup153 binds one Ran molecule in apparently non-allosteric fashion. The affinity is measurably higher for RanGDP than for RanGTP and varies modestly between the individual zinc fingers. By microcalorimetric and mutational analysis, we determined that one specific hydrogen bond accounts for most of the differences in the binding affinity of individual zinc fingers. Genomic analysis reveals that only in animals do NPCs contain Ran-binding zinc fingers. We speculate that these organisms evolved a mechanism to maintain a high local concentration of Ran at the vicinity of the NPC, using this zinc finger domain as a sink.
Figures
Figure 1
Alignment of the four Ran-binding zinc fingers of Nup153 from R. norvegicus. The numbering used for individual zinc fingers is listed above. Identical residues are highlighted in dark blue, with decreasing levels of conservation highlighted in lighter colors of blue. Below is the consensus sequence for the“RanBP2” family of zinc finger proteins.
Figure 2
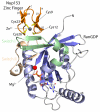
Ribbon diagram representation of the Nup153-ZnF2•RanGDP complex. RanGDP β-sheets are colored darkblue with α-helices colored light blue. The Nup153-ZnF molecule is colored orange. Both the switch I and switch II regions of Ran are highlighted. Representative of the “RanBP2” family of protein-binding zinc fingers, the Nup153 ZnF contains two orthogonal β-hairpins with four cysteine residues, colored yellow, to coordinate a single Zn2+ ion colored grey. The zinc finger binds near the switch I region of RanGDP, indicated here by the secondary structural element β2. The GDP nucleotide is shown in the center of Ran with a bound Mg2+ ion.
Figure 3
Crystallographic lattice formed in the crystallization of a Nup153-ZnF•RanGDP complex. Residues highlighted in red indicate contacts made between neighboring Ran molecules down a single plane of the P21 lattice. This plane represents the smallest crystallographic interface and was chosen for crystal engineering to stabilize packing, in hopes of improving diffraction. The enlarged 3D image on the right depicts residues binding at the interface. Thr32' and His53 make a single hydrogen bond, mirrored across a 2-fold symmetrical face as indicated. A clash between Phe35' and Pro58, though tolerated, was theorized to partially counteract the strong interaction between Thr32' and His53. A point-mutation of Phe35' to Serine stabilizes the crystal contact and increases the resolution of our crystallographic experiments by 1 Å.
Figure 4
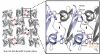
Comparison of the four individual Nup153 zinc fingers (ZnFs), colored orange, in complex with RanGDP, colored blue. The four individual ZnFs are overlaid to highlight differences and similarities in binding with Ran. Residues that make intermolecular hydrogen bonds are colored in green, while residues that facilitate hydrophobic interactions are colored violet. (a) A overview of the Nup153-ZnF•RanGDP complex showing that each ZnF binds independently and at the same location with RanGDP. Hydrogen bonds conserved amongst the four ZnFs are labeled. The variable hydrogen bond at residue 8ZnF with Gln10Ran is only formed between Ran and ZnF1 or ZnF3. (b) An enlarged view of all residues responsible for the hydrophobic interaction at the Nup153-ZnF•RanGDP interface. (c) An enlarged view of hydrogen bonds, in green, that facilitate the Nup153-ZnF•RanGDP interaction. Conserved H-bonds are made between the carbonyl of Cys26ZnF with the sidechain hydroxyl group of Thr42Ran, and Lys38Ran with the carbonyl at residue10ZnF. A variable H-bond is present at residue 8ZnF binding with Gln10Ran. A stable bond is formed between Gln8ZnF1 and Gln10Ran, as well as between Glu8ZnF3 and Gln10Ran. Residue 8 of ZnF2 and ZnF4, colored dark-blue, contain an Asp residue, too short to make the H-bond with Gln10Ran. (d) A conserved water network at the interface between Nup153-ZnF and RanGDP. Residues facilitating these contacts are labeled and highlighted in green, with water molecules colored violet. (e) A more detailed view of the non-conserved hydrogen bond made with Gln10Ran by Gln8ZnF1 and Glu8ZnF3, colored green. Asp8ZnF4 and Asp8ZnF4, colored blue, are unable to bridge the distance necessary to make the H-bond with Gln10Ran, a calculated distance of ∼5.0 Å.
Figure 4
Comparison of the four individual Nup153 zinc fingers (ZnFs), colored orange, in complex with RanGDP, colored blue. The four individual ZnFs are overlaid to highlight differences and similarities in binding with Ran. Residues that make intermolecular hydrogen bonds are colored in green, while residues that facilitate hydrophobic interactions are colored violet. (a) A overview of the Nup153-ZnF•RanGDP complex showing that each ZnF binds independently and at the same location with RanGDP. Hydrogen bonds conserved amongst the four ZnFs are labeled. The variable hydrogen bond at residue 8ZnF with Gln10Ran is only formed between Ran and ZnF1 or ZnF3. (b) An enlarged view of all residues responsible for the hydrophobic interaction at the Nup153-ZnF•RanGDP interface. (c) An enlarged view of hydrogen bonds, in green, that facilitate the Nup153-ZnF•RanGDP interaction. Conserved H-bonds are made between the carbonyl of Cys26ZnF with the sidechain hydroxyl group of Thr42Ran, and Lys38Ran with the carbonyl at residue10ZnF. A variable H-bond is present at residue 8ZnF binding with Gln10Ran. A stable bond is formed between Gln8ZnF1 and Gln10Ran, as well as between Glu8ZnF3 and Gln10Ran. Residue 8 of ZnF2 and ZnF4, colored dark-blue, contain an Asp residue, too short to make the H-bond with Gln10Ran. (d) A conserved water network at the interface between Nup153-ZnF and RanGDP. Residues facilitating these contacts are labeled and highlighted in green, with water molecules colored violet. (e) A more detailed view of the non-conserved hydrogen bond made with Gln10Ran by Gln8ZnF1 and Glu8ZnF3, colored green. Asp8ZnF4 and Asp8ZnF4, colored blue, are unable to bridge the distance necessary to make the H-bond with Gln10Ran, a calculated distance of ∼5.0 Å.
Figure 5
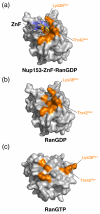
Surface representation of RanGDP or RanGTP, grey, with or without Nup153-ZnF,blue ribbon diagram. Residues involved in intermolecular contact are colored orange. (a) ZnF•RanGDP complex. Lys38Ran and Thr42Ran, located in the switch I region, form conserved H-bonds with the ZnF, and are, known to undergo significant conformational changes upon binding of GTP. Both residues are positioned to interact with ZnF in this conformation. (b) RanGDP interaction surface in the absence of ZnF. (c) RanGTP in absence of modeled ZnF. Lys38Ran and Thr42Ran are displaced up to 30 Å, and are no longer able to interact with the Nup153-ZnF in this conformation.
Figure 6
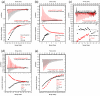
Isothermal calorimetry (ITC) for each individual ZnF binding RanGDP. Point-mutations are used to probe the variable H-bond interaction with Gln10Ran, determined to be the interaction responsible for discrepancies in affinities measured between ZnFs and RanGDP. Data for independent ZnFs is fit to a single site model with _N_=1. (a) WT-ZnF1 shown in red compared with mutated ZnF1 in black. Mutating Gln6 to Asp is shown to have a minor effect on binding with Gln10Ran. (b) WT-ZnF2 shown in red with the mutant protein shown in black. WT-ZnF2 shows no detectable signal, however by mutating Asp6 to Gln signal is restored. (c) WT-ZnF3 is shown in red and mutant ZnF3 is shown in black. Mutating Glu6 to Asp is enough to abolish signal by disrupting a single H-bond with Gln10Ran. (d) WT-ZnF4 is shown in red and has no detectable signal. However mutating Asp6 to Gln introduces a new H-bond with Gln10Ran and restores signal, similar to results seen with ZnF2. (e) Binding of the WT Nup153-ZnF domain to GDP (in red) is compared to binding with RanGTP (in black). In agreement with our structural observations, RanGDP binds ZnFs with strong and weak sites, while RanGTP binds with the same stoichiometry, but only with weak affinity. Experimental values for N, Kd, enthalpy, ΔH, and entropy, TΔS, are listed in Table 2. Constructs are described in Supplementary Table 1.
Figure 7
ITC illustrating low μM affinity between RanGDP and two tandem ZnF pairs from the Nup153-and Nup358-ZnF domain. (a) Interaction between RanGDP and ZnF12. The data is best fit to a two-site model suggesting each ZnF binds independently, but with different affinities. (b) Interaction between ZnF34 and RanGDP, again suggesting two independent sites with different affinities. (c) Interaction between Nup358ZnF12 and RanGDP. As with Nup153, the ZnFs of Nup358 bind with low μM affinity to RanGDP. The data is best fit to a single site model in accordance with both ZnFs from Nup358 lacking the residue nececcary at pos8 to H-bond with Gln10Ran. Experimental values for N, Kd, enthalpy, ΔH, and entropy, TΔS, are listed in Table 2. Constructs are described in Supplementary Table 1.
Figure 8
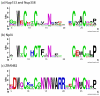
Weblogos from members of the RanBP-2 class of zinc fingers known to have unique binding partners. The logos highlight similarities and differences amongst the three groups of zinc fingers: the Ran binding Nup153/Nup358 group, the ubiquitin binding Npl4 group, and the single-stranded RNA binding zn256 group. The logos demonstrate the overall conservation of the canonical RanBP2 type zinc finger sequence W-X-C-X(2,4)-C-X(3)-N-X(6)-C-X(2)-C, while highlighting regions important for facilitating binding with a unique binding partner. (a) ZnFs from Nup153 and Nup358 that bind with Ran. Only the first finger from each protein has been used in the alignment. The previously described “LVA,” in positions 13, 14, and 25, have low information content, although position 25 has been shown influence Ran binding . (b) The logo for Npl4 again demonstrates the elevated conservation of positions 13, 14, and 15, previously shown to modulate interaction with ubiquitin. (c) The ZRANB2 logo highlights the strong conservation this sequence in eukaryotes. A structure of ZRANB2 (PDB codes 3G9Y and 2K1P), demonstrates the strict conservation and elevated level of positive charges at positions 14-20 that regulate binding of single-stranded RNA . Each logo is based on alignments, shown in Fig S2, of sequences from a diverse group of eukaryotes.
Similar articles
- Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules.
Prunuske AJ, Liu J, Elgort S, Joseph J, Dasso M, Ullman KS. Prunuske AJ, et al. Mol Biol Cell. 2006 Feb;17(2):760-9. doi: 10.1091/mbc.e05-06-0485. Epub 2005 Nov 28. Mol Biol Cell. 2006. PMID: 16314393 Free PMC article. - The crystal structure of the Ran-Nup153ZnF2 complex: a general Ran docking site at the nuclear pore complex.
Schrader N, Koerner C, Koessmeier K, Bangert JA, Wittinghofer A, Stoll R, Vetter IR. Schrader N, et al. Structure. 2008 Jul;16(7):1116-25. doi: 10.1016/j.str.2008.03.014. Structure. 2008. PMID: 18611384 - Molecular characterization of the Ran-binding zinc finger domain of Nup153.
Higa MM, Alam SL, Sundquist WI, Ullman KS. Higa MM, et al. J Biol Chem. 2007 Jun 8;282(23):17090-100. doi: 10.1074/jbc.M702715200. Epub 2007 Apr 10. J Biol Chem. 2007. PMID: 17426026 - On the asymmetric partitioning of nucleocytoplasmic transport - recent insights and open questions.
Kalita J, Kapinos LE, Lim RYH. Kalita J, et al. J Cell Sci. 2021 Apr 1;134(7):jcs240382. doi: 10.1242/jcs.240382. Epub 2021 Apr 13. J Cell Sci. 2021. PMID: 33912945 Review. - Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease.
Jamali T, Jamali Y, Mehrbod M, Mofrad MR. Jamali T, et al. Int Rev Cell Mol Biol. 2011;287:233-86. doi: 10.1016/B978-0-12-386043-9.00006-2. Int Rev Cell Mol Biol. 2011. PMID: 21414590 Review.
Cited by
- Nuclear transport proteins: structure, function, and disease relevance.
Yang Y, Guo L, Chen L, Gong B, Jia D, Sun Q. Yang Y, et al. Signal Transduct Target Ther. 2023 Nov 10;8(1):425. doi: 10.1038/s41392-023-01649-4. Signal Transduct Target Ther. 2023. PMID: 37945593 Free PMC article. Review. - Encephalomyocarditis virus Leader protein hinge domain is responsible for interactions with Ran GTPase.
Bacot-Davis VR, Palmenberg AC. Bacot-Davis VR, et al. Virology. 2013 Aug 15;443(1):177-85. doi: 10.1016/j.virol.2013.05.002. Epub 2013 May 25. Virology. 2013. PMID: 23711384 Free PMC article. - REV7 has a dynamic adaptor region to accommodate small GTPase RAN/Shigella IpaB ligands, and its activity is regulated by the RanGTP/GDP switch.
Wang X, Pernicone N, Pertz L, Hua D, Zhang T, Listovsky T, Xie W. Wang X, et al. J Biol Chem. 2019 Oct 25;294(43):15733-15742. doi: 10.1074/jbc.RA119.010123. Epub 2019 Sep 4. J Biol Chem. 2019. PMID: 31484720 Free PMC article. - The nuclear pore complex has entered the atomic age.
Brohawn SG, Partridge JR, Whittle JR, Schwartz TU. Brohawn SG, et al. Structure. 2009 Sep 9;17(9):1156-68. doi: 10.1016/j.str.2009.07.014. Structure. 2009. PMID: 19748337 Free PMC article. Review. - Architecture of the cytoplasmic face of the nuclear pore.
Bley CJ, Nie S, Mobbs GW, Petrovic S, Gres AT, Liu X, Mukherjee S, Harvey S, Huber FM, Lin DH, Brown B, Tang AW, Rundlet EJ, Correia AR, Chen S, Regmi SG, Stevens TA, Jette CA, Dasso M, Patke A, Palazzo AF, Kossiakoff AA, Hoelz A. Bley CJ, et al. Science. 2022 Jun 10;376(6598):eabm9129. doi: 10.1126/science.abm9129. Epub 2022 Jun 10. Science. 2022. PMID: 35679405 Free PMC article.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, Rout MP. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
- Kiseleva E, Allen TD, Rutherford S, Bucci M, Wente SR, Goldberg MW. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J Struct Biol. 2004;145:272–88. - PubMed
- Kiseleva E, Rutherford S, Cotter LM, Allen TD, Goldberg MW. Steps of nuclear pore complex disassembly and reassembly during mitosis in early Drosophila embryos. J Cell Sci. 2001;114:3607–18. - PubMed
- Schwartz TU. Modularity within the architecture of the nuclear pore complex. Curr Opin Struct Biol. 2005;15:221–6. - PubMed
- Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 GM068762/GM/NIGMS NIH HHS/United States
- R01 GM077537/GM/NIGMS NIH HHS/United States
- R01 GM077537-03/GM/NIGMS NIH HHS/United States
- GM68762/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous