The cytokine interleukin-33 mediates anaphylactic shock - PubMed (original) (raw)
The cytokine interleukin-33 mediates anaphylactic shock
Peter N Pushparaj et al. Proc Natl Acad Sci U S A. 2009.
Retraction in
- Retraction. The cytokine interleukin-33 mediates anaphylactic shock.
Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, Liew FY. Pushparaj PN, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13877. doi: 10.1073/pnas.1212463109. Epub 2012 Aug 13. Proc Natl Acad Sci U S A. 2012. PMID: 22891328 Free PMC article. No abstract available.
Abstract
Anaphylactic shock is characterized by elevated immunoglobulin-E (IgE) antibodies that signal via the high affinity Fc epsilon receptor (Fc epsilonRI) to release inflammatory mediators. Here we report that the novel cytokine interleukin-33 (IL-33) potently induces anaphylactic shock in mice and is associated with the symptom in humans. IL-33 is a new member of the IL-1 family and the ligand for the orphan receptor ST2. In humans, the levels of IL-33 are substantially elevated in the blood of atopic patients during anaphylactic shock, and in inflamed skin tissue of atopic dermatitis patients. In murine experimental atopic models, IL-33 induced antigen-independent passive cutaneous and systemic anaphylaxis, in a T cell-independent, mast cell-dependent manner. In vitro, IL-33 directly induced degranulation, strong eicosanoid and cytokine production in IgE-sensitized mast cells. The molecular mechanisms triggering these responses include the activation of phospholipase D1 and sphingosine kinase1 to mediate calcium mobilization, Nuclear factor-kappaB activation, cytokine and eicosanoid secretion, and degranulation. This report therefore reveals a hitherto unrecognized pathophysiological role of IL-33 and suggests that IL-33 may be a potential therapeutic target for anaphylaxis, a disease of considerable unmet medical need.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
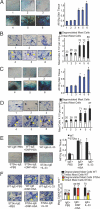
Fig. 1.
IL-33 in atopic patients during anaphylaxis and inflammation. (A) Total IgE levels in healthy donors, atopic controls, and atopic patients with anaphylactic shock. (B) IL-33 levels in healthy donors, atopic controls, and atopic patients with anaphylactic shock. Data are mean ± SD, n = 5 for each group. *P < 0.01 compared to healthy. (C) Total RNA was extracted from skin biopsy samples of healthy donors and skin lesions of atopic dermatitis patients. IL-33 mRNA was determined by quantitative polymerase chain reaction (Q-PCR). Data are mean ± SD, n = 7, *P < 0.01 compared to healthy skin. (D) Immune staining with anti-IL-33 antibody or control IgG. Pictures are representative of skin biopsy samples of seven healthy and seven dermatitis donors.
Fig. 2.
Passive cutaneous anaphylaxis (PCA). PCA was induced as described in Materials and Methods. (A–D) Mice were treated as follows: Treatment 1, injected with PBS alone; 2, sensitized with IgE alone; 3, challenged with IL-33 alone; 4, sensitized with IgE and challenged with DNP/HSA; 5, sensitized with IgE and challenged with IL-33; and 6, sensitized with IgE and challenged with DNP/HSA+IL-33. (A, B) In wild-type (WT) mice, IL-33 alone did not induce PCA (group 3) but induced PCA and mast cell degranulation in IgE-sensitized mice (group 5). IL-33 enhanced antigen (DNP-HSA) induced PCA and mast cell degranulation (group 6). (C, D) IL-33 induced PCA and mast cell degranulation in RAG1−/− mice as efficiently as in WT mice. (E, F) Mice were treated as follows: WT sensitized with IgE and challenged with PBS (WT-IgE+PBS), WT sensitized with IgE and challenged with antigen (WT-IgE+DNP-HSA), WT sensitized with IgE and challenged with IL-33 (WT-IgE+IL-33); ST2−/− mice sensitized with IgE and challenged with PBS (ST2ko-IgE+PBS), ST2−/− sensitized with IgE and challenged with antigen (ST2ko-IgE+DNP-HSA), ST2−/− sensitized with IgE and challenged with IL-33 (ST2ko-IgE+IL-33). IL-33 induced PCA in WT but not in ST2−/− mice. Panels B, D, and F are toluidine blue stained samples and degranulated mast cells are indicated by arrows. Results in A–F are means ± SD, n = 6. *P < 0.01, compared with control mice injected with PBS alone or IgE+PBS.
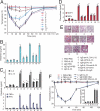
Fig. 3.
Systemic anaphylactic shock in mice. For systemic anaphylactic shock in wild-type mice, mice were injected i.v. with the various reagents as indicated in the figure and in Materials and Methods (treatments 1–11). (A) Rectal temperature was measured every 10 minutes after challenge. (B) Serum histamine, (C) plasma cytokines and (D) chemokines were examined at 120 minutes after challenge. Data are mean ± SEM, n = 5, *P < 0.01 compared to group 1, +P < 0.01 compared with group 5. (E) Lung sections from mice injected i.v. with the various reagents as indicated in the figure and in Materials and Methods (treatments 1–11), were stained with hematoxylin and eosin and examined under light microscopy. The pictures are representative of five mice per group. For full treatment details, please refer to the method, “Induction and Measurement of Anaphylactic Shock.” (F) To test the specificity of IL-33–induced systemic anaphylactic shock, ST2−/− mice were injected i.v. with the various reagents as indicated in the figure and in Materials and Methods (treatments 1–6), data are mean ± SEM, n = 5, *P < 0.01 compared with group 1.
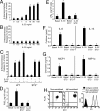
Fig. 4.
IL-33 triggers mast cell degranulation and proinflammatory mediators in an ST2-dependent manner. (A) Wild-type mast cells were sensitized with IgE and β-hexosaminidase release was measured after 30 minutes of stimulation with increasing concentration of IL-33. (B) Wild-type mast cells (nonsensitized) were stimulated with increasing concentrations of IL-33, and β-hexosaminidase release was measured after 30 minutes of stimulation. (C) Wild-type and ST2−/− mast cells were sensitized with IgE and β-hexosaminidase release was measured after 30 minutes of stimulation with: 10 ng/ml of IL-33; or 1 μg/ml of antigen DNP-HSA or a combination of both triggers. PGD2 (D), LTB4 (E), cytokines (F), and chemokines (G) production was determined 24 hours after 10 ng/ml of IL-33 stimulation or from wild-type and ST2−/− mast cells. Results are means ± SD, n = 3, and from three independent experiments, *P < 0.01 compared to basal level. (H) Cell surface expression levels of FcεRI and ST2 on nonsensitized and IgE-sensitized murine mast cells. Results are representative from three independent experiments.
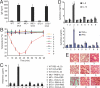
Fig. 5.
Effect of IL-33 on ovalbumin (OVA) sensitized mice. (A) OVA induces similar levels of IgE in wild-type (WT), mast cell–deficient (MC−/−) and ST2-null (ST2−/−) mice. (B–E) Mice were treated as follows: treatment 1, WT mice sensitized with PBS and challenged with IL-33; 2, WT mice sensitized with OVA and challenged with PBS; 3, WT mice sensitized with OVA and challenged with IL-33; 4, MC−/− mice sensitized with PBS and challenged with IL-33; 5, MC−/− mice sensitized with OVA and challenged with PBS; 6, MC−/− mice sensitized with OVA and challenged with IL-33; 7, ST2−/− mice sensitized with PBS and challenged with IL-33; 8, ST2−/− mice sensitized with OVA and challenged with PBS; 9, ST2−/− mice sensitized with OVA and challenged with IL-33. (B) Rectal temperature was measured every 10 minutes after IL-33 challenge (C) Serum histamine, (D) plasma cytokines were examined at 120 minutes after challenge. Data are mean ± SEM, n = 5, *P < 0.01 compared to group 1, +P < 0.01 compared with group 5. (E) Lung sections from mice euthanized at 120 minutes after challenge were stained with hematoxylin and eosin and examined under light microscopy. Pictures are representative of five mice per group.
Similar articles
- NecroX-5 suppresses IgE/Ag-stimulated anaphylaxis and mast cell activation by regulating the SHP-1-Syk signaling module.
Li X, Kwon O, Kim DY, Taketomi Y, Murakami M, Chang HW. Li X, et al. Allergy. 2016 Feb;71(2):198-209. doi: 10.1111/all.12786. Epub 2015 Nov 23. Allergy. 2016. PMID: 26456627 - Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation.
Lu Y, Yang JH, Li X, Hwangbo K, Hwang SL, Taketomi Y, Murakami M, Chang YC, Kim CH, Son JK, Chang HW. Lu Y, et al. Biochem Pharmacol. 2011 Dec 1;82(11):1700-8. doi: 10.1016/j.bcp.2011.08.022. Epub 2011 Sep 3. Biochem Pharmacol. 2011. PMID: 21907188 - IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells.
Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, Stassen M, Oyoshi MK, Finkelman FD, Geha RS. Galand C, et al. J Allergy Clin Immunol. 2016 Nov;138(5):1356-1366. doi: 10.1016/j.jaci.2016.03.056. Epub 2016 Jun 2. J Allergy Clin Immunol. 2016. PMID: 27372570 Free PMC article. - Mast Cells and Anaphylaxis.
Lieberman P, Garvey LH. Lieberman P, et al. Curr Allergy Asthma Rep. 2016 Mar;16(3):20. doi: 10.1007/s11882-016-0598-5. Curr Allergy Asthma Rep. 2016. PMID: 26857018 Review. - Impact of mast cells on the skin.
Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P, Toniato E, Pantalone A, Neri G, Frydas S, Rosati M, Tei M, Speziali A, Saggini R, Pandolfi F, Cerulli G, Theoharides TC, Conti P. Kritas SK, et al. Int J Immunopathol Pharmacol. 2013 Oct-Dec;26(4):855-9. doi: 10.1177/039463201302600403. Int J Immunopathol Pharmacol. 2013. PMID: 24355220 Review.
Cited by
- Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease.
Zhang Y, Liu H, Wang L, Yang F, Hu Y, Ren X, Li G, Yang Y, Sun S, Li Y, Chen X, Li X, Jin Q. Zhang Y, et al. PLoS One. 2013 Jun 28;8(6):e67430. doi: 10.1371/journal.pone.0067430. Print 2013. PLoS One. 2013. PMID: 23840697 Free PMC article. - Disease severity in K/BxN serum transfer-induced arthritis is not affected by IL-33 deficiency.
Martin P, Talabot-Ayer D, Seemayer CA, Vigne S, Lamacchia C, Rodriguez E, Finckh A, Smith DE, Gabay C, Palmer G. Martin P, et al. Arthritis Res Ther. 2013 Jan 16;15(1):R13. doi: 10.1186/ar4143. Arthritis Res Ther. 2013. PMID: 23324173 Free PMC article. - Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages.
Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, Das A, Hogaboam CM. Joshi AD, et al. BMC Immunol. 2010 Oct 19;11:52. doi: 10.1186/1471-2172-11-52. BMC Immunol. 2010. PMID: 20958987 Free PMC article. - Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP.
Choi HW, Brooking-Dixon R, Neupane S, Lee CJ, Miao EA, Staats HF, Abraham SN. Choi HW, et al. Immunity. 2013 Dec 12;39(6):1108-20. doi: 10.1016/j.immuni.2013.11.009. Immunity. 2013. PMID: 24332031 Free PMC article. - A protective role for inflammasome activation following injury.
Osuka A, Hanschen M, Stoecklein V, Lederer JA. Osuka A, et al. Shock. 2012 Jan;37(1):47-55. doi: 10.1097/SHK.0b013e318234f7ff. Shock. 2012. PMID: 21921832 Free PMC article.
References
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
- O'Neill LA, Dinarello CA. The IL-1 receptor/Toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. - PubMed
- Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol. 1998;161:4866–4874. - PubMed
- Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G9818261/MRC_/Medical Research Council/United Kingdom
- MC_U105178805/MRC_/Medical Research Council/United Kingdom
- G0801198/MRC_/Medical Research Council/United Kingdom
- G0700794/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases