The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons - PubMed (original) (raw)
. 2009 Jul 1;122(Pt 13):2274-82.
doi: 10.1242/jcs.048975. Epub 2009 Jun 9.
Affiliations
- PMID: 19509051
- PMCID: PMC2723145
- DOI: 10.1242/jcs.048975
The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons
Jufang Chang et al. J Cell Sci. 2009.
Abstract
NIMA-related kinases (Neks) belong to a large family of Ser/Thr kinases that have critical roles in coordinating microtubule dynamics during ciliogenesis and mitotic progression. The Nek kinases are also expressed in neurons, whose axonal projections are, similarly to cilia, microtubule-abundant structures that extend from the cell body. We therefore investigated whether Nek kinases have additional, non-mitotic roles in neurons. We found that Nek3 influences neuronal morphogenesis and polarity through effects on microtubules. Nek3 is expressed in the cytoplasm and axons of neurons and is phosphorylated at Thr475 located in the C-terminal PEST domain, which regulates its catalytic activity. Although exogenous expression of wild-type or phosphomimic (T475D) Nek3 in cultured neurons has no discernible impact, expression of a phospho-defective mutant (T475A) or PEST-truncated Nek3 leads to distorted neuronal morphology with disturbed polarity and deacetylation of microtubules via HDAC6 in its kinase-dependent manner. Thus, the phosphorylation at Thr475 serves as a regulatory switch that alters Nek3 function. The deacetylation of microtubules in neurons by unphosphorylated Nek3 raises the possibility that it could have a role in disorders where axonal degeneration is an important component.
Figures
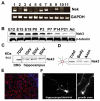
Fig. 1.
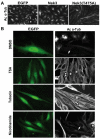
Nek3 is expressed in neuronal cells and is a cytoplasmic enzyme. (A) The transcripts of Nek1, Nek3, Nek6, Nek7 and Nek9 are readily detected by RT-PCR in purified mouse DRG neurons cultured for 14 days. (B) Lysates of brain isolated from mice of the indicated age examined by western blotting using anti-Nek3 antibodies (BD Transduction Labs) to determine Nek3 levels. (C) Western blot showing that Nek3 is present in glia-free neuronal cultures from DRG or hippocampus. DIV, days in vitro. (D) Western blot of lysates prepared from whole DRG (Total), cell bodies after axotomy (Body) and severed axons (Axon). Schematic diagram of a single DRG neuronal ganglion with red box depicting the site of axotomy is shown on the left. (E) Immunofluorescent microscopy of Nek3 in E15 DRG. Endogeneous Nek3 is primarily located in the cytoplasm. Red, Nek3; Blue, nucleus counterstained with Hoechst dye 33258. (F) Immunofluorescent microscopy with anti-Myc antibody of cultured hippocampal and DRG neurons expressing Myc-tagged wild-type Nek3. Nek3 is present in the neuronal soma, axonal shaft and growth cones.
Fig. 2.
Nek3 is constitutively phosphorylated at Thr475. (A) Lysates from HeLa cells expressing Myc-tagged wild-type Nek3 or Nek3(T475A) were immunoprecipitated with Myc antibody and immunoblotted with antibodies to phosphothreonine (pThr) or Nek3. Only wild-type Nek3 was detected with the Thr-P antibody. (B) Lysates from HeLa cells expressing indicated Nek3 proteins were probed with Nek3 Thr475-_P_-specific antibody (pNek3Thr475). Only wild-type Nek3 and kdNek3 are recognized by this antibody. (C) HeLa cell lysates containing wild-type Nek3 incubated with calf intestinal alkaline phosphatase (CIP) and analyzed by western blot. (D) Peptide competition assay to assess Nek3 Thr475-_P_-specific antibody specificity. Lysate from HeLa cells expressing wild-type Nek3 was analyzed by western blotting using Nek3 Thr475-_P_-specific antibody that had been preincubated with Nek3 phosphorylated peptide (the immunogen), its non-phosphorylated counterpart, or no peptide (control). No signal is detected when the Nek3 Thr475-_P_-specific antibody was preincubated with the phosphorylated peptide.
Fig. 3.
Nek3 activity is regulated by the PEST domain and phosophorylation of Thr475. (A) Schematic diagram depicting important features of Nek3 and the constructs used in this study. Empty rectangle represents the conserved kinase domain of NIMA-related proteins; Filled boxes in C-terminus are PEST domains; single asterisk represents Thr475 and double asterisks, Asp143, in the ATP-binding site required for kinase activity. (B) Immunofluorescence images of cultured DRG neurons expressing Myc-tagged wild-type Nek3, Nek3-T1, Nek3-T2, Nek3(T475A) and Nek3(T475D) stained with anti-Myc antibody. All Nek3 proteins were located within the cytoplasm. Neurons expressing Nek3-T1, Nek3-T2 and Nek3(T475A) show distorted cellular morphology and aberrant neurite outgrowth (multiple processes).
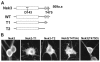
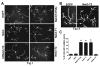
Fig. 4.
Nek3 mutants disrupt neuronal polarity. (A) Hippocampal neurons infected with lentivirus expressing EGFP, wild-type Nek3 or Nek3 mutants were stained with neuron-specific β III tubulin (Tuj1) antibody to highlight their morphology. A single long process was observed in neurons expressing EGFP, wild-type Nek3 or kinase-dead (kd)Nek3-T2. However, neurons expressing Nek3(T475A), Nek3-T1 or Nek3-T2 have multiple long processes indicative of aberrant polarity. (B) Immunohistochemical analysis using the Tau-1 axonal marker demonstrating that the multiple processes induced by Nek3-T2 are axons (arrows; cell body is denoted by arrowhead). In addition, the Tau-1-stained processes display morphology typical of axons with a very fine caliber near the nucleus and gradually increasing caliber toward the end. (C) Quantification of hippocampal neuronal polarity. Neurons were cultured as above and the number of processes per neuron were counted. Approximately 200 neurons expressing each of the indicated proteins were analyzed per experiment (_n_=3 independent experiments). Neurons extending a single axon were defined as polarized. Nek3(T475A), Nek3-T1 and Nek3-T2 induced aberrant polarity in hippocampal neurons; however, an active kinase was required to induce the formation of multiple processes (see kdNek3-T2). Results are mean ± s.d. *P<0.0001 (vs EGFP control).
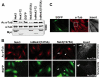
Fig. 5.
Nek3 mutants induce α-tubulin deacetylation. (A) The level of α-tubulin acetylation in lysates from DRG neurons expressing the indicated Nek3 proteins was analyzed by western blotting using anti-Ac-tubulin antibody. Neurons expressing Nek3-T2 and Nek3(T475A) have low levels of Ac-tubulin. Total α-tubulin (α-tub) levels are equivalent in all samples. Myc antibody was used to examine Nek3 protein levels. (B) HeLa cells transfected with constructs expressing wild-type Nek3, Nek3(T475A) or kdNek3(T475A) and stained with anti-Ac-tubulin (red). EGFP signal indicates transfected cells (green). An untransfected cell with normal acetylated microtubule network is indicated with an arrow. Nek3(T475A)-transfected cells show an almost complete lack of acetylated microtubules (arrowhead and asterisk). Cells expressing wild-type Nek3 or kdNek3(T475A) are indistinguishable from untransfected cells. Insert is an enlarged view of the acetylated microtubule network in untransfected and Nek3(T475A)-transfected cells. (C) HeLa cells transfected with Nek3(T475A) stained for total α-tubulin (red) to examine the microtubule network independent of its acetylation state. EGFP (green) indicates transfected cells. The area of the enlarged view (insert) is indicated by an asterisk. Note that most untransfected cells have a rounded morphology, whereas T475A-transfected cells display an elongated shape. No differences in the microtubule network were observed in transfected versus untransfected cells (insert).
Fig. 6.
Nek3-regulated α-tubulin deacetylation requires HDAC6. (A) HeLa cells expressing EGFP, wild-type Nek3 or Nek3(T475A) stained for Ac-tubulin 48 hours after transfection. Note that cells expressing Nek3(T475A) have reduced Ac-tubulin levels and are configured in an elongated, aligned manner. Cells expressing wild-type Nek3 are indistinguishable from control cells expressing GFP. (B) Nek3(T475A)-expressing HeLa cells treated with trichostatin A (TSA) (500 nM), tubacin (20 μM), nicotinamide (20 mM) or DMSO (control) and stained with anti-Ac-tubulin antibody to assess the level of acetylated microtubules. DMSO- and nicotinamide-treated cells display the characteristic robust reduction in microtubule acetylation mediated by Nek3(T475A) (compare cells denoted by arrow and asterisk in DMSO- and nicotinamide-treated cells). Cells treated with the broad HDAC inhibitor TSA or the HDAC6-specific inhibitor tubacin have normal levels of acetylated α-tubulin [compare adjacent untransfected cell (two asterisks) with Nek3(T475A)-expressing cell (one asterisk)], indicating that these inhibitors rescue the phenotypes induced by Nek3(T475A). All images were taken with the same exposure time.
Similar articles
- Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts.
Sudo H, Baas PW. Sudo H, et al. J Neurosci. 2010 May 26;30(21):7215-26. doi: 10.1523/JNEUROSCI.0048-10.2010. J Neurosci. 2010. PMID: 20505088 Free PMC article. - HDAC6 is a microtubule-associated deacetylase.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. Hubbert C, et al. Nature. 2002 May 23;417(6887):455-8. doi: 10.1038/417455a. Nature. 2002. PMID: 12024216 - HDAC6 inhibition results in tau acetylation and modulates tau phosphorylation and degradation in oligodendrocytes.
Noack M, Leyk J, Richter-Landsberg C. Noack M, et al. Glia. 2014 Apr;62(4):535-47. doi: 10.1002/glia.22624. Epub 2014 Jan 24. Glia. 2014. PMID: 24464872 - Structure, function, and evolution of plant NIMA-related kinases: implication for phosphorylation-dependent microtubule regulation.
Takatani S, Otani K, Kanazawa M, Takahashi T, Motose H. Takatani S, et al. J Plant Res. 2015 Nov;128(6):875-91. doi: 10.1007/s10265-015-0751-6. Epub 2015 Sep 9. J Plant Res. 2015. PMID: 26354760 Review. - The NEK family of serine/threonine kinases as a biomarker for cancer.
Panchal NK, Evan Prince S. Panchal NK, et al. Clin Exp Med. 2023 Feb;23(1):17-30. doi: 10.1007/s10238-021-00782-0. Epub 2022 Jan 17. Clin Exp Med. 2023. PMID: 35037094 Review.
Cited by
- "Stop Ne(c)king around": How interactomics contributes to functionally characterize Nek family kinases.
Meirelles GV, Perez AM, de Souza EE, Basei FL, Papa PF, Melo Hanchuk TD, Cardoso VB, Kobarg J. Meirelles GV, et al. World J Biol Chem. 2014 May 26;5(2):141-60. doi: 10.4331/wjbc.v5.i2.141. World J Biol Chem. 2014. PMID: 24921005 Free PMC article. Review. - Identification of candidate genetic variants and altered protein expression in neural stem and mature neural cells support altered microtubule function to be an essential component in bipolar disorder.
Truvé K, Parris TZ, Vizlin-Hodzic D, Salmela S, Berger E, Ågren H, Funa K. Truvé K, et al. Transl Psychiatry. 2020 Nov 9;10(1):390. doi: 10.1038/s41398-020-01056-1. Transl Psychiatry. 2020. PMID: 33168801 Free PMC article. - Disrupted myelination network in the cingulate cortex of Parkinson's disease.
Xie S, Yang J, Huang S, Fan Y, Xu T, He J, Guo J, Ji X, Wang Z, Li P, Chen J, Zhang Y. Xie S, et al. IET Syst Biol. 2022 May;16(3-4):98-119. doi: 10.1049/syb2.12043. Epub 2022 Apr 8. IET Syst Biol. 2022. PMID: 35394697 Free PMC article. - Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts.
Sudo H, Baas PW. Sudo H, et al. J Neurosci. 2010 May 26;30(21):7215-26. doi: 10.1523/JNEUROSCI.0048-10.2010. J Neurosci. 2010. PMID: 20505088 Free PMC article. - Identification of interphase functions for the NIMA kinase involving microtubules and the ESCRT pathway.
Govindaraghavan M, McGuire Anglin SL, Shen KF, Shukla N, De Souza CP, Osmani SA. Govindaraghavan M, et al. PLoS Genet. 2014 Mar 27;10(3):e1004248. doi: 10.1371/journal.pgen.1004248. eCollection 2014 Mar. PLoS Genet. 2014. PMID: 24675878 Free PMC article.
References
- Araki, T., Sasaki, Y. and Milbrandt, J. (2004). Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010-1013. - PubMed
- Arama, E., Yanai, A., Kilfin, G., Bernstein, A. and Motro, B. (1998). Murine NIMA-related kinases are expressed in patterns suggesting distinct functions in gametogenesis and a role in the nervous system. Oncogene 16, 1813-1823. - PubMed
- Badano, J. L., Mitsuma, N., Beales, P. L. and Katsanis, N. (2006). The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125-148. - PubMed
- Ballif, B. A., Villen, J., Beausoleil, S. A., Schwartz, D. and Gygi, S. P. (2004). Phosphoproteomic analysis of the developing mouse brain. Mol. Cell Proteomics 3, 1093-1101. - PubMed
- Belham, C., Roig, J., Caldwell, J. A., Aoyama, Y., Kemp, B. E., Comb, M. and Avruch, J. (2003). A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J. Biol. Chem. 278, 34897-34909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG13730/AG/NIA NIH HHS/United States
- P20 NS057105/NS/NINDS NIH HHS/United States
- CA111966/CA/NCI NIH HHS/United States
- NS040745/NS/NINDS NIH HHS/United States
- K08NS055980/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases