Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo - PubMed (original) (raw)
Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo
Honglu Zhang et al. Cancer Res. 2009.
Abstract
Signal transduction modifiers that modulate the lysophosphatidic acid (LPA) pathway have potential as anticancer agents. Herein, we describe metabolically stabilized LPA analogues that reduce cell migration and invasion and cause regression of orthotopic breast tumors in vivo. Two diastereoisomeric alpha-bromophosphonates (BrP-LPA) were synthesized, and the pharmacology was determined for five LPA G protein-coupled receptors (GPCRs). The syn and anti diastereomers of BrP-LPA are pan-LPA GPCR antagonists and are also nanomolar inhibitors of the lysophospholipase D activity of autotaxin, the dominant biosynthetic source of LPA. Computational models correctly predicted the diastereoselectivity of antagonism for three GPCR isoforms. The anti isomer of BrP-LPA was more effective than syn isomer in reducing migration of MDA-MB-231 cells, and the anti isomer was superior in reducing invasion of these cells. Finally, orthotopic breast cancer xenografts were established in nude mice by injection of MB-231 cells in an in situ cross-linkable extracellular matrix. After 2 weeks, mice were treated with the BrP-LPA alone (10 mg/kg), Taxol alone (10 mg/kg), or Taxol followed by BrP-LPA. All treatments significantly reduced tumor burden, and BrP-LPA was superior to Taxol in reducing blood vessel density in tumors. Moreover, both the anti- and syn-BrP-LPA significantly reduced tumors at 3 mg/kg.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
G. Tigyi is an equity holder in RxBio. G.D. Prestwich is an equity holder in Glycosan BioSystems and advisor to RxBio and Echelon Biosciences. The other authors declared no potential conflicts of interest.
Figures
Figure 1
A, structures of LPA and stabilized analogues. B, synthesis of _syn_- and _anti_-BrP-LPA isomers 1a and 1b. See text for experimental details.
Figure 2
Docked complexes of _syn_-1a and _anti_-1b in LPA1-3 accurately predict pharmacology. Syn -1a and anti -1b are ball-and-stick models, receptor residues are labeled stick models, and ribbons show the protein backbone (red, amino terminus; blue, carboxy terminus). A, typical view shows the syn isomer 1a in the LPA2 receptor, with the ligand positioned at the interface between the transmembrane α-helical segments and the extracellular loops (top). B and C, close views of _syn_-1a and anti-1b, respectively, docked in the LPA1-3 receptors as viewed from the extracellular side. Interactions differing significantly between the complex of each receptor with 1a and 1b have green lines.
Figure 3
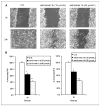
Effect of syn-1a and anti-1b BrP-LPA diastereomers on MDA-MB-231 cell migration. A, confluent MDA-MB-231 cells were scratched and then treated with syn-1a or anti-1b (10 and 40 μmol/L) and compared with untreated cells (CTL) at 24 h. B, quantification for the _anti_-BrP-LPA 1b and _syn_-BrP-LPA 1a. Asterisks indicate significant differences from control (CTL) at P < 0.0005 (*) and P < 0.0001 (**) for syn isomer 1a and at P < 0.001 (*) and P < 0.0001 (**) for _anti_-isomer 1b.
Figure 4
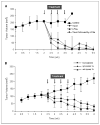
BrP-LPA treatment reduces tumor size in orthotopic breast cancer xenografts. A, effect of BrP-LPA 1 treatment (10 mg/kg i.p.) on MDA-MB-231 tumor growth in vivo. At 6 wk, all tumor volumes in treated animals were significantly different from the controls but not different from each other (P < 0.05). B, effect of _syn_-BrP-LPA 1a and _anti_-BrP-LPA 1b treatment (3 mg/kg i.p.) on MDA-MB-231 tumor growth. At 6 wk, 1a and 1b were statistically different from controls (P < 0.05) but not from each other (P = 0.16).
Figure 5
BrP-LPA decreases tumor size and vascularity. A, difference in gross tumor size. H&E staining (B) and CD31-specific staining (C) show relative angiogenesis. D, quantification of newly generated vessels in the tumor samples. The asterisk (*) indicates that Taxol alone and Taxol then BrP-LPA 1 (mixed isomers) were statistically different from the control (P < 0.05) but not different from each other. Treatment with BrP-LPA 1 alone showed significantly lower blood vessel density (**) than the controls (P < 0.001) or either Taxol treatment (P < 0.05).
Similar articles
- Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors.
Xu X, Yang G, Zhang H, Prestwich GD. Xu X, et al. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):140-6. doi: 10.1016/j.prostaglandins.2009.07.006. Epub 2009 Aug 12. Prostaglandins Other Lipid Mediat. 2009. PMID: 19682598 Free PMC article. Review. - Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells.
Gaetano CG, Samadi N, Tomsig JL, Macdonald TL, Lynch KR, Brindley DN. Gaetano CG, et al. Mol Carcinog. 2009 Sep;48(9):801-9. doi: 10.1002/mc.20524. Mol Carcinog. 2009. PMID: 19204929 Free PMC article. - Synthesis, pharmacology, and cell biology of sn-2-aminooxy analogues of lysophosphatidic acid.
Gajewiak J, Tsukahara R, Fujiwara Y, Tigyi G, Prestwich GD. Gajewiak J, et al. Org Lett. 2008 Mar 20;10(6):1111-4. doi: 10.1021/ol7030747. Epub 2008 Feb 20. Org Lett. 2008. PMID: 18284246 Free PMC article. - Therapeutic potential of autotaxin/lysophospholipase d inhibitors.
Federico L, Pamuklar Z, Smyth SS, Morris AJ. Federico L, et al. Curr Drug Targets. 2008 Aug;9(8):698-708. doi: 10.2174/138945008785132439. Curr Drug Targets. 2008. PMID: 18691016 Free PMC article. Review.
Cited by
- Autotaxin in Breast Cancer: Role, Epigenetic Regulation and Clinical Implications.
Drosouni A, Panagopoulou M, Aidinis V, Chatzaki E. Drosouni A, et al. Cancers (Basel). 2022 Nov 4;14(21):5437. doi: 10.3390/cancers14215437. Cancers (Basel). 2022. PMID: 36358855 Free PMC article. Review. - Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine.
Prestwich GD. Prestwich GD. J Control Release. 2011 Oct 30;155(2):193-9. doi: 10.1016/j.jconrel.2011.04.007. Epub 2011 Apr 14. J Control Release. 2011. PMID: 21513749 Free PMC article. Review. - Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression.
Panupinthu N, Lee HY, Mills GB. Panupinthu N, et al. Br J Cancer. 2010 Mar 16;102(6):941-6. doi: 10.1038/sj.bjc.6605588. Br J Cancer. 2010. PMID: 20234370 Free PMC article. Review. - KAI1/CD82 gene and autotaxin-lysophosphatidic acid axis in gastrointestinal cancers.
Wang S, Chen J, Guo XZ. Wang S, et al. World J Gastrointest Oncol. 2022 Aug 15;14(8):1388-1405. doi: 10.4251/wjgo.v14.i8.1388. World J Gastrointest Oncol. 2022. PMID: 36160748 Free PMC article. Review. - Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer.
Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, Albaugh M, Vidal-Vanaclocha F, Palmieri D, Barbier M, Murone M, Steeg PS. Marshall JC, et al. J Natl Cancer Inst. 2012 Sep 5;104(17):1306-19. doi: 10.1093/jnci/djs319. Epub 2012 Aug 21. J Natl Cancer Inst. 2012. PMID: 22911670 Free PMC article.
References
- Drees BE, Mills GB, Rommel C, Prestwich GD. Therapeutic potential of phosphoinositide 3-kinase inhibitors. Expert Opin Ther Patents. 2004;14:703–32.
- Maira S-M, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidyl 3-kinase/mammalian target of rapamycin inhbitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. - PubMed
- Feng L, Mills GB, Prestwich GD. Modulators of lysophosphatidic acid signaling. Expert Opin Ther Patents. 2003;13:1619–34.
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–91. - PubMed
- Umezu-Goto M, Tanyi J, Lahad J, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92:1115–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS29632/NS/NINDS NIH HHS/United States
- R01 NS029632/NS/NINDS NIH HHS/United States
- HL61469/HL/NHLBI NIH HHS/United States
- R01 CA092160/CA/NCI NIH HHS/United States
- R01 HL061469/HL/NHLBI NIH HHS/United States
- HL070231/HL/NHLBI NIH HHS/United States
- CA921160/CA/NCI NIH HHS/United States
- R01 CA092160-10/CA/NCI NIH HHS/United States
- R01 HL070231/HL/NHLBI NIH HHS/United States
- HL0084007/HL/NHLBI NIH HHS/United States
- R01 HL084007/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous