Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease - PubMed (original) (raw)
Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease
Wolfgang J Streit et al. Acta Neuropathol. 2009 Oct.
Abstract
The role of microglial cells in the pathogenesis of Alzheimer's disease (AD) neurodegeneration is unknown. Although several works suggest that chronic neuroinflammation caused by activated microglia contributes to neurofibrillary degeneration, anti-inflammatory drugs do not prevent or reverse neuronal tau pathology. This raises the question if indeed microglial activation occurs in the human brain at sites of neurofibrillary degeneration. In view of the recent work demonstrating presence of dystrophic (senescent) microglia in aged human brain, the purpose of this study was to investigate microglial cells in situ and at high resolution in the immediate vicinity of tau-positive structures in order to determine conclusively whether degenerating neuronal structures are associated with activated or with dystrophic microglia. We used a newly optimized immunohistochemical method for visualizing microglial cells in human archival brain together with Braak staging of neurofibrillary pathology to ascertain the morphology of microglia in the vicinity of tau-positive structures. We now report histopathological findings from 19 humans covering the spectrum from none to severe AD pathology, including patients with Down's syndrome, showing that degenerating neuronal structures positive for tau (neuropil threads, neurofibrillary tangles, neuritic plaques) are invariably colocalized with severely dystrophic (fragmented) rather than with activated microglial cells. Using Braak staging of Alzheimer neuropathology we demonstrate that microglial dystrophy precedes the spread of tau pathology. Deposits of amyloid-beta protein (Abeta) devoid of tau-positive structures were found to be colocalized with non-activated, ramified microglia, suggesting that Abeta does not trigger microglial activation. Our findings also indicate that when microglial activation does occur in the absence of an identifiable acute central nervous system insult, it is likely to be the result of systemic infectious disease. The findings reported here strongly argue against the hypothesis that neuroinflammatory changes contribute to AD dementia. Instead, they offer an alternative hypothesis of AD pathogenesis that takes into consideration: (1) the notion that microglia are neuron-supporting cells and neuroprotective; (2) the fact that development of non-familial, sporadic AD is inextricably linked to aging. They support the idea that progressive, aging-related microglial degeneration and loss of microglial neuroprotection rather than induction of microglial activation contributes to the onset of sporadic Alzheimer's disease. The results have far-reaching implications in terms of reevaluating current treatment approaches towards AD.
Figures
Fig. 1
Anti-iba1 immunohistochemistry produces specific staining of microglia in archival human brain tissue. a, b Low power views demonstrate abundance and even spacing of microglial cells throughout the cortical gray matter of the entorhinal cortex in a non-pathological control subject (case no. 3; Table 1). Pial surface is on far left in a. All cells show a non-activated, non-dystrophic morphology typical for normal, resting microglia. c, d Comparison of resting and activated microglial morphology in two age-matched individuals (case nos. 2 and 6, respectively). Activated microglia (d) show cytoplasmic hypertrophy in a subject with sepsis. Scale bars 500 μm (a), 100 μm (b), 50 μm (c, d)
Fig. 2
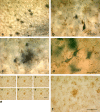
Microglial fragmentation is widespread in Alzheimer’s disease brain and colocalized with tau pathology. Double-label immunohistochemistry for microglia (iba1) and tau (AT8) in the entorhinal cortex (EC) of case no. 12 (a–c). a Low power view reveals tau-positive neuritic plaques (black spots) in the cortical gray matter and diffusely distributed neuropil threads and tangles (lower right); microglia (brown) are evenly distributed throughout. Higher magnification shows neurofibrillary tangle and neuropil threads (b) and neuritic plaque (c), which are surrounded by fragmented microglia lacking intact morphology. d Ghost tangles in the EC shown with silver impregnation are surrounded by microglial fragments. e–f iba 1 staining shows detail of fragmented microglial cells in a cluster (center) and in surrounding cells. e Focus series of individual micrographs taken 10–15 μm apart, reassembled into composite image in f. Scale bars 100 μm (a, b, e, f), 50 μm (c, d)
Fig. 3
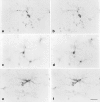
Microglial degeneration can occur independent of age and is evident in cases of young subjects with minimal tau pathology. Double-label immunohistochemistry for microglia (iba1) and tau (AT8) is shown in the hippocampus of two young subjects with no (b) or minimal (d) tau pathology (case nos. 1, 5). a, c Focus series of four individual micrographs taken 10–15 μm apart, and reassembled into composite images in b and d. Note the difference in microglial morphology in the two young subjects, one of which shows minimal tau pathology evident as neuropil threads (arrows in d). Microglia in b show normal ramified appearance but are fragmented in d. Scale bars 20 μm (a, c); 10 μm (b, d)
Fig. 4
Single-label (iba1) staining of microglia in two subjects with Down’s syndrome (case nos. 14, 15) is shown in a and b, respectively. Both micrographs reveal a total loss of microglial cell integrity and show presence of microglial cell debris throughout; middle temporal gyrus (a) and entorhinal cortex (b). Scale bar 50 μm
Fig. 5
Microglial fragmentation precedes the spread of tau pathology in the temporal lobe. Double-label immunohistochemistry for microglia (iba1) and tau (AT8) is shown in three subjects with tau pathology increasing from Braak stages 0–III (case nos. 3, 8, and 9). Camera lucida drawings of the actual sections are shown in c, f, and i indicating for orientation the uncus, as well as both sampling areas in the EC and middle temporal gyrus (MTG); areas of tau pathology are shaded in orange. Representative micrographs of the EC (a, d, g) and MTG (b, e, h) reveal microglia (brown) and tau pathology (black) at the different stages. Normal ramified microglia are evident at stage 0 in both EC and MTG in the absence of tau pathology (a, b); mostly fragmented microglia are seen in association with a neurofibrillary tangle and neuropil threads in d, whereas mostly ramified and only a single fragmented cell (arrow) are present in e during stage I; severe microglial fragmentation and loss of discernable cell shape is colocalized with extensive tau pathology in g; microglial processes are fragmented also in h in the absence of neurodegeneration, but cells retain recognizable contours. Scale bar 50 μm (a, b, d, e, g, h)
Fig. 6
Microglial activation is absent in the presence of Aβ but extensive during sepsis. a Double-label immunohistochemistry for microglia (iba1) and Aβ (4G8) reveals fully ramified, non-activated microglia evenly distributed throughout the EC gray matter in the presence of extensive Aβ deposits (case no. 17). Higher magnification reveals full extent of microglial ramification when Aβ is visualized using silver staining in the same subject (b). c Fully activated and hypertrophic microglia are present throughout the EC gray and white matter in a subject with sepsis (case no. 6). Inset Detail of microglial hypertrophy typical for activated microglia. Scale bars 100 μm (a), 50 μm (b), 500 μm (c), 50 μm (inset)
Fig. 7
Shown are representative examples of dystrophic (a, b; case no. 9), resting (c, d; case no. 3), activated (e, f; case no. 6) microglial cells at high power. Each set of images shows the same microscopic field in two focal planes using differential interference optics. Dystrophic cells are readily distinguished from both resting and activated cells by their fragmented cytoplasmic processes. Activated cells are distinguished by their hypertrophy. Both resting and activated cells have continuous, non-fragmented processes. All images are from entorhinal cortex. Scale bar 20 μm
Similar articles
- Microglial pathology in Down syndrome.
Xue QS, Streit WJ. Xue QS, et al. Acta Neuropathol. 2011 Oct;122(4):455-66. doi: 10.1007/s00401-011-0864-5. Epub 2011 Aug 17. Acta Neuropathol. 2011. PMID: 21847625 - The relationship between the morphological subtypes of microglia and Alzheimer's disease neuropathology.
Paasila PJ, Davies DS, Kril JJ, Goldsbury C, Sutherland GT. Paasila PJ, et al. Brain Pathol. 2019 Nov;29(6):726-740. doi: 10.1111/bpa.12717. Epub 2019 Mar 22. Brain Pathol. 2019. PMID: 30803086 Free PMC article. - Microglial activation occurs late during preclinical Alzheimer's disease.
Streit WJ, Braak H, Del Tredici K, Leyh J, Lier J, Khoshbouei H, Eisenlöffel C, Müller W, Bechmann I. Streit WJ, et al. Glia. 2018 Dec;66(12):2550-2562. doi: 10.1002/glia.23510. Epub 2018 Nov 11. Glia. 2018. PMID: 30417428 - Dystrophic microglia in late-onset Alzheimer's disease.
Streit WJ, Khoshbouei H, Bechmann I. Streit WJ, et al. Glia. 2020 Apr;68(4):845-854. doi: 10.1002/glia.23782. Epub 2020 Jan 10. Glia. 2020. PMID: 31922322 Review. - Alzheimer's disease and amyloid: culprit or coincidence?
Skaper SD. Skaper SD. Int Rev Neurobiol. 2012;102:277-316. doi: 10.1016/B978-0-12-386986-9.00011-9. Int Rev Neurobiol. 2012. PMID: 22748834 Review.
Cited by
- Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain.
Verkhratsky A, Augusto-Oliveira M, Pivoriūnas A, Popov A, Brazhe A, Semyanov A. Verkhratsky A, et al. Pflugers Arch. 2021 May;473(5):753-774. doi: 10.1007/s00424-020-02465-3. Epub 2020 Sep 26. Pflugers Arch. 2021. PMID: 32979108 Review. - From development to dysfunction: microglia and the complement cascade in CNS homeostasis.
Zabel MK, Kirsch WM. Zabel MK, et al. Ageing Res Rev. 2013 Jun;12(3):749-56. doi: 10.1016/j.arr.2013.02.001. Epub 2013 Feb 16. Ageing Res Rev. 2013. PMID: 23419464 Free PMC article. Review. - Microglial senescence in neurodegeneration: Insights, implications, and therapeutic opportunities.
Samuel Olajide T, Oyerinde TO, Omotosho OI, Okeowo OM, Olajide OJ, Ijomone OM. Samuel Olajide T, et al. Neuroprotection. 2024 Sep;2(3):182-195. doi: 10.1002/nep3.56. Epub 2024 Sep 15. Neuroprotection. 2024. PMID: 39364217 Free PMC article. - Dynamic neuroinflammatory profiles predict Alzheimer's disease pathology in microglia-containing cerebral organoids.
Kuhn MK, Kang RY, Kim C, Tagay Y, Morris N, Tabdanov ED, Elcheva IA, Proctor EA. Kuhn MK, et al. bioRxiv [Preprint]. 2024 Apr 3:2023.11.16.567220. doi: 10.1101/2023.11.16.567220. bioRxiv. 2024. PMID: 38014053 Free PMC article. Preprint. - Interferon-γ blocks signalling through PDGFRβ in human brain pericytes.
Jansson D, Scotter EL, Rustenhoven J, Coppieters N, Smyth LC, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Dragunow M. Jansson D, et al. J Neuroinflammation. 2016 Sep 21;13(1):249. doi: 10.1186/s12974-016-0722-4. J Neuroinflammation. 2016. PMID: 27654972 Free PMC article.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF02815208', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf02815208'}, {'type': 'PubMed', 'value': '8871945', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8871945/'}\]}
- Aisen PS (1996) Inflammation and Alzheimer disease. Mol Chem Neuropathol 28:83–88 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1212/01.wnl.0000313813.48505.86', 'is_inner': False, 'url': 'https://doi.org/10.1212/01.wnl.0000313813.48505.86'}, {'type': 'PubMed', 'value': '18519870', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18519870/'}\]}
- Arvanitakis Z, Grodstein F, Bienias JL et al (2008) Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 70:2219–2225 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1750-3639.2008.00134.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1750-3639.2008.00134.x'}, {'type': 'PMC', 'value': 'PMC8095633', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC8095633/'}, {'type': 'PubMed', 'value': '18363937', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18363937/'}\]}
- Boche D, Nicoll JA (2008) The role of the immune system in clearance of Abeta from the brain. Brain Pathol 18:267–278 - PMC - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF00308809', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf00308809'}, {'type': 'PubMed', 'value': '1759558', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1759558/'}\]}
- Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1750-3639.1991.tb00661.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1750-3639.1991.tb00661.x'}, {'type': 'PubMed', 'value': '1669710', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1669710/'}\]}
- Braak H, Braak E (1991) Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1:213–216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical