Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS - PubMed (original) (raw)
Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS
Stephen P J Fancy et al. Genes Dev. 2009.
Abstract
The progressive loss of CNS myelin in patients with multiple sclerosis (MS) has been proposed to result from the combined effects of damage to oligodendrocytes and failure of remyelination. A common feature of demyelinated lesions is the presence of oligodendrocyte precursors (OLPs) blocked at a premyelinating stage. However, the mechanistic basis for inhibition of myelin repair is incompletely understood. To identify novel regulators of OLP differentiation, potentially dysregulated during repair, we performed a genome-wide screen of 1040 transcription factor-encoding genes expressed in remyelinating rodent lesions. We report that approximately 50 transcription factor-encoding genes show dynamic expression during repair and that expression of the Wnt pathway mediator Tcf4 (aka Tcf7l2) within OLPs is specific to lesioned-but not normal-adult white matter. We report that beta-catenin signaling is active during oligodendrocyte development and remyelination in vivo. Moreover, we observed similar regulation of Tcf4 in the developing human CNS and lesions of MS. Data mining revealed elevated levels of Wnt pathway mRNA transcripts and proteins within MS lesions, indicating activation of the pathway in this pathological context. We show that dysregulation of Wnt-beta-catenin signaling in OLPs results in profound delay of both developmental myelination and remyelination, based on (1) conditional activation of beta-catenin in the oligodendrocyte lineage in vivo and (2) findings from APC(Min) mice, which lack one functional copy of the endogenous Wnt pathway inhibitor APC. Together, our findings indicate that dysregulated Wnt-beta-catenin signaling inhibits myelination/remyelination in the mammalian CNS. Evidence of Wnt pathway activity in human MS lesions suggests that its dysregulation might contribute to inefficient myelin repair in human neurological disorders.
Figures
Figure 1.
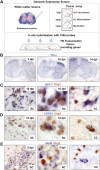
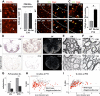
Whole-genome screen for TFs dynamically expressed during remyelination. (A) Diagram illustrating strategy for whole-genome in situ expression-based screen for TF-encoding genes induced during remyelination in adult CNS. (B) Thra, encoding a receptor for thyroid hormone and known factor associated with OLP differentiation, is an example of a factor showing dynamic expression with a sharp peak at 10 dpl when OLP differentiation commences in this lesion model. (C,D) Examples of factors expressed exclusively in Olig2-positive oligodendrocyte lineage cells during remyelination in the spinal cord (SC) include Spi2.2 (C) and COPS3 (D), which also showed similar expression patterns during remyelination of mouse corpus callosum (CC). (E) Other examples including MafB were expressed in nonoligodendrocyte lineage cells.
Figure 2.
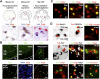
Tcf4 (Tcf7l2) is expressed in oligodendrocyte lineage during remyelination of adult brain and spinal cord. (A) Expression of Tcf4 (Tcf7l2) transcripts in three models of CNS remyelination induced by lysolecithin injection in mouse spinal cord (SC), dietary cuprizone in mouse corpus callosum (CC), and EtBr injection in rat CCP. (B) Tcf4 protein is expressed in white matter (WM) of SC during developmental myelination beginning at P1 and during remyelination of focal demyelination in adult, but is not expressed in the white matter of normal unlesioned adult SC. Low-power whole lesion images are shown of Tcf4 with Olig2 and DAPI/Olig2 during remyelination in the adult. (C) Tcf4 protein is expressed in a subset of Olig2-positive cells within remyelinating areas of lysolecithin-induced demyelination in adult mouse SC, at 5 dpl, 10 dpl, and 14 dpl. (D) Tcf4 protein expression was confirmed at 10 dpl in EtBr-induced demyelination in adult rat CCP. At 10 dpl in this model, Tcf4 was coexpressed in cells that expressed Nkx2.2 message, PDGFRα message, and nuclear Olig1 protein (which we demonstrated previously identifies OLPs) (Kitada and Rowitch 2006), but Tcf4 expression identified Olig2 cells that segregated from expression of the mature oligodendrocyte marker PLP-exon 3b, as well as cytoplasmic Olig1 protein (which we showed previously identifies mature oligodendrocytes) (Arnett et al. 2004; Kitada and Rowitch 2006). Tcf4 colabels a subset of Tle1 (Groucho)-expressing cells in 10-dpl CCP (yellow arrowhead).
Figure 3.
Tcf4 (Tcf7l2) is expressed in oligodendrocyte lineage during developmental myelination. (A) Tcf4 labels a subset of Olig2 cells at P1 and P15 during developmental myelination of spinal cord white matter. (B) At P1, Olig1 protein is almost entirely nuclear (which we showed previously is a marker for OLPs) (Kitada and Rowitch 2006), and Tcf4 is expressed in a subset of these cells. (C) Tcf4 protein does not colocalize with cytoplasmic Olig1 protein (which we showed previously marks mature oligodendrocytes) (Kitada and Rowitch 2006) at P9. (D) Tcf4 colocalizes with OLP marker PDGFRα protein at P5 but does not colocalize with mature oligodendrocyte marker PLP message at P5 or P9.
Figure 4.
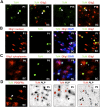
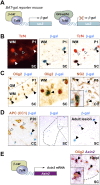
Use of BAT-gal mice demonstrates that Tcf4 functions in catenin-dependent Wnt signaling during brain and spinal cord developmental myelination and remyelination of adult spinal cord. (A) Schematic for Tcf4–β-catenin-driven β-galactosidase (β-gal) reporter expression in BAT-gal mice. (C) β-gal is expressed in a subset of Olig2 cells in BAT-gal reporter mice in presumptive white matter only of P1 SC, and in a subset of NG2 cells in P5 CC, suggesting that the Wnt pathway is active in these cells. β-gal is expressed in a subset of Tcf4 cells in BAT-gal Wnt reporter mice P1 SC white matter (B), but is not expressed in cells expressing the Wnt pathway inhibitor APC (CC1) in P20 CC (D). (D) β-gal is re-expressed in a demyelinated lesion at 5 dpl following injection of lysolecithin into BAT-gal spinal cord. Filled arrowheads refer to BAT-gal-positive cells. Unfilled arrowheads show Tcf4 or CC1 staining cells that are BAT-gal-negative. (E) Schematic for Tcf4–β-catenin-driven Axin-2 mRNA expression. Axin2 is a known target of Wnt signaling (Lustig et al. 2002) that is globally up-regulated by activation of the Wnt pathway. Endogenous Axin-2 mRNA is expressed in a subset of Olig2 cells at 10 dpl in a remyelinating spinal cord lesion.
Figure 5.
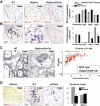
Overexpression of activated β-catenin in oligodendrocyte lineage cells during development leads to hypomyelination and a significant delay in OLPs to oligodendrocyte differentiation, without affecting the embryonic production of premyelinating OLP. (A) Overexpression of activated β-catenin in oligodendrocyte lineage cells in Olig2cre/DA-Cat mice does not affect the production of premyelinating PDGFRα-expressing OLPs (arrows) in spinal cord at developmental stage E18. (B) Tcf4 protein is not expressed in the spinal cords of wild-type (WT) or Olig2cre/DA-Cat mice at E18, indicating that the Tcf4/activated β-catenin complex does not affect embryonic production of oligodendrocyte lineage cells, but appears at P1 both in the presumptive white matter in a subset of Olig2 cells (arrowheads). (C) At P15 there is a reduction in the number of Olig2 cells in spinal cord of the Olig2cre/DA-Cat, and Tcf4 is expressed in a higher proportion [(*) P = 3.8 × 10−5) of the Olig2-expressing cells. (D,G) Olig2cre/DA-Cat mice show a marked reduction [(G) (*) P = 0.0002 at P9; (**) P = 5.5 × 10−5 at P15] in the number of mature oligodendrocytes expressing PLP message during myelination at P9 and P15, with a clear reduction in the white matter visible under dark field (DF) at P15. (E,H) Ataxia and tremor are associated with a significant hypomyelination in Olig2cre/DA-Cat mice, as seen by EM in the P15 spinal cord white matter, and a significant (P = 1.9 × 10−35) reduction in the myelin thickness as assessed by G-ratio analysis. (F,I) The hypomyelination during developmental myelination in Olig2cre/DA-Cat represents a delay in OLP differentiation rather than an irreversible block; myelin appearance and G-ratio are similar to wild-type littermates in the adult P50 white matter.
Figure 6.
Tcf4 is expressed in oligodendrocyte lineage in human developmental white matter and in active areas of MS lesions. (A) Tcf4 is expressed in white matter tracts during myelination of human developmental brain at postnatal age 1 mo, 3.5 mo, and 16 mo, but is not expressed by 7 yr. Tcf4 colocalizes with Olig2 when expressed in the developing human corpus callosum. (B) Tcf4 protein expression is evident in active MS lesions, but it is not seen in normal-appearing white matter (NAWM) or in the core of chronic MS lesions. An illustrative MS case is shown with several lesion types present. NAWM stains with Luxol Fast Blue (LFB) and contains sparse LN3(HLA-DR)-positive inflammatory cells, organized SMI-31 axon fibers, and no Tcf4-positive cells. Chronic plaques have sparse LFB staining and LN3-positive cells, intact axons, but no Tcf4-positive cells. In contrast, Tcf4-positive cells are present in active areas of plaques with abundant LN3-positive cells and intact demyelinated axons. Tcf4 expression in active lesions colocalizes (open arrowheads) with a subset of Olig2 cells.
Figure 7.
Overexpression of activated β-catenin in oligodendrocyte lineage cells during remyelination in the adult causes a delay in repair due to a delay in OLP differentiation, without affecting their recruitment into the lesion. (A) Similar numbers of OLPs expressing Nkx2.2 protein, are present within remyelinating adult spinal cord lesions at 10 dpl in wild type (WT), Olig2cre control, and Olig2cre/DA-Cat mice, and also at 5 dpl and 14 dpl in wild type (gray bars), Olig2cre control (white bars), and_Olig2cre/DA-Cat_ mice (black bars). (B) There is a significant reduction [(*) P = 0.028 at 10 dpl; (**) P = 0.0009 at 14 dpl] in the subsequent differentiation of recruited OLPs into mature remyelinating oligodendrocytes expressing PLP message during remyelination in the Olig2cre/DA-Cat (black bars) compared with wild type (gray bars) or Olig2cre controls (white bars). (C) Delay in OLP differentiation during remyelination results in thinner myelin sheaths at 14 dpl in Olig2cre/DA-Cat mice evident by EM, and with significant (P = 4.6 × 10−9) change in G-ratio. (D) APCmin mice, lacking one allele of the Wnt pathway inhibitor APC, show a marked deficit in remyelination of adult spinal cord at 14 dpl, due to a significant reduction [(*) P = 0.012] of differentiation into mature _PLP_-expressing oligodendrocytes, despite normal recruitment of Nkx2.2-expressing OLPs.
Figure 8.
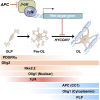
Scheme indicating expression of Tcf4 proteins and effects of Wnt–β-catenin signaling to inhibit the transition from premyelinating to mature oligodendrocyte. Our genetic evidence is consistent with APC (CC1, a known marker of mature oligodendrocytes) acting to promote differentiation. HYCCIN, a Wnt-repressed target (Kawasoe et al. 2000) with essential roles in human myelination (Zara et al. 2006), is expressed in rodent oligodendrocytes and down-regulated in Olig2cre/DA-Cat mice (Supplemental Fig. 8). Temporal expression of Tcf4 and markers of oligodendrocyte lineage tested in this study is shown. (Pre OL) Premyelinating oligodendrocyte; (OL) oligodendrocyte.
Comment in
- Modulating myelination: knowing when to say Wnt.
Rosenberg SS, Chan JR. Rosenberg SS, et al. Genes Dev. 2009 Jul 1;23(13):1487-93. doi: 10.1101/gad.1824009. Genes Dev. 2009. PMID: 19571178 Free PMC article.
Similar articles
- The Wnt effector transcription factor 7-like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/β-catenin signaling.
Hammond E, Lang J, Maeda Y, Pleasure D, Angus-Hill M, Xu J, Horiuchi M, Deng W, Guo F. Hammond E, et al. J Neurosci. 2015 Mar 25;35(12):5007-22. doi: 10.1523/JNEUROSCI.4787-14.2015. J Neurosci. 2015. PMID: 25810530 Free PMC article. - Myelin transcription factor 1 (Myt1) expression in demyelinated lesions of rodent and human CNS.
Vana AC, Lucchinetti CF, Le TQ, Armstrong RC. Vana AC, et al. Glia. 2007 May;55(7):687-97. doi: 10.1002/glia.20492. Glia. 2007. PMID: 17330875 Free PMC article. - Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination.
Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Fancy SP, et al. Nat Neurosci. 2011 Jun 26;14(8):1009-16. doi: 10.1038/nn.2855. Nat Neurosci. 2011. PMID: 21706018 Free PMC article. - The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.
Keirstead HS, Blakemore WF. Keirstead HS, et al. Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review. - Wnt signaling in remyelination in multiple sclerosis: friend or foe?
Xie C, Li Z, Zhang GX, Guan Y. Xie C, et al. Mol Neurobiol. 2014 Jun;49(3):1117-25. doi: 10.1007/s12035-013-8584-6. Epub 2013 Nov 16. Mol Neurobiol. 2014. PMID: 24243343 Review.
Cited by
- NG2-glia and their functions in the central nervous system.
Dimou L, Gallo V. Dimou L, et al. Glia. 2015 Aug;63(8):1429-51. doi: 10.1002/glia.22859. Epub 2015 May 24. Glia. 2015. PMID: 26010717 Free PMC article. Review. - Adenomatous polyposis coli regulates radial axonal sorting and myelination in the PNS.
Elbaz B, Traka M, Kunjamma RB, Dukala D, Brosius Lutz A, Anton ES, Barres BA, Soliven B, Popko B. Elbaz B, et al. Development. 2016 Jul 1;143(13):2356-66. doi: 10.1242/dev.135913. Epub 2016 May 25. Development. 2016. PMID: 27226321 Free PMC article. - Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration.
Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJ, Lubetzki C. Moyon S, et al. J Neurosci. 2015 Jan 7;35(1):4-20. doi: 10.1523/JNEUROSCI.0849-14.2015. J Neurosci. 2015. PMID: 25568099 Free PMC article. - PRC2 Acts as a Critical Timer That Drives Oligodendrocyte Fate over Astrocyte Identity by Repressing the Notch Pathway.
Wang W, Cho H, Kim D, Park Y, Moon JH, Lim SJ, Yoon SM, McCane M, Aicher SA, Kim S, Emery B, Lee JW, Lee S, Park Y, Lee SK. Wang W, et al. Cell Rep. 2020 Sep 15;32(11):108147. doi: 10.1016/j.celrep.2020.108147. Cell Rep. 2020. PMID: 32937136 Free PMC article. - Contribution of the oligodendrocyte lineage to CNS repair and neurodegenerative pathologies.
Tognatta R, Miller RH. Tognatta R, et al. Neuropharmacology. 2016 Nov;110(Pt B):539-547. doi: 10.1016/j.neuropharm.2016.04.026. Epub 2016 Apr 21. Neuropharmacology. 2016. PMID: 27108096 Free PMC article. Review.
References
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJM, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. - PubMed
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. - PubMed
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2005;5:997–1014. - PubMed
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases