Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment - PubMed (original) (raw)
. 2009 Jul 9;460(7252):259-63.
doi: 10.1038/nature08099. Epub 2009 Jun 10.
Affiliations
- PMID: 19516257
- PMCID: PMC2831539
- DOI: 10.1038/nature08099
Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment
Olaia Naveiras et al. Nature. 2009.
Abstract
Osteoblasts and endothelium constitute functional niches that support haematopoietic stem cells in mammalian bone marrow. Adult bone marrow also contains adipocytes, the number of which correlates inversely with the haematopoietic activity of the marrow. Fatty infiltration of haematopoietic red marrow follows irradiation or chemotherapy and is a diagnostic feature in biopsies from patients with marrow aplasia. To explore whether adipocytes influence haematopoiesis or simply fill marrow space, we compared the haematopoietic activity of distinct regions of the mouse skeleton that differ in adiposity. Here we show, by flow cytometry, colony-forming activity and competitive repopulation assay, that haematopoietic stem cells and short-term progenitors are reduced in frequency in the adipocyte-rich vertebrae of the mouse tail relative to the adipocyte-free vertebrae of the thorax. In lipoatrophic A-ZIP/F1 'fatless' mice, which are genetically incapable of forming adipocytes, and in mice treated with the peroxisome proliferator-activated receptor-gamma inhibitor bisphenol A diglycidyl ether, which inhibits adipogenesis, marrow engraftment after irradiation is accelerated relative to wild-type or untreated mice. These data implicate adipocytes as predominantly negative regulators of the bone-marrow microenvironment, and indicate that antagonizing marrow adipogenesis may enhance haematopoietic recovery in clinical bone-marrow transplantation.
Figures
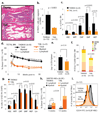
Figure 1. Hematopoietic stem cells and progenitors are reduced in number, frequency and cycling capacity in adipocyte-rich bone marrow during homeostasis
**a.**H&E-stain of decalcified thoracic vertebra (top) and fourth-tail-segment (bottom), 12-week-old C57BL/6J mice. **b.**Absolute number of hematopoietic cells (CD45+) per vertebral segment. **c.**Progenitor frequency within the hematopoietic compartment (CD45+). **d.**Competitive engraftment (250,000 CD45.1 tail or thorax BM; 250,000 CD45.2 competitor BM), **e.**Day 13 spleen-colony assay, and **f.**CFU-progenitor assay from tail and thorax BM. **g.**Progenitor cell cycle analysis .average % cells in S/G2/M transition ± SEM. **h.**100 tail and thorax BM sorted HSC (ckit+Lin-Sca1+Flk2−; >95% purity) were transplanted competitively, then engraftment in peripheral blood monitored. **i.**CD34 expression within the HSC fraction (KLSF, ckit+Lin-Sca1+Flk2−); % CD34low within KLSF fraction indicated.
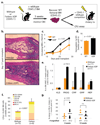
Figure 2. The lack of bone marrow adipocytes post-irradiation in fatless mice enhances hematopoietic progenitor expansion and post-transplant recovery
**a.**Experimental design. Wildtype FVB or fatless FVB.A-ZIP/F 16-week-old mice (CD45.1) were lethally irradiated and transplanted with 200,000 CD45.2, MHC-compatible DBA/1 wild-type BM. Femurs were isolated on day 17–20 post-transplant and donor DBA CD45.2 wildtype BM was recovered by high purity FACS, then used for progenitor assays or competitive serial transplantation. **b.**Femoral H&E in the third week post-transplant. **c.**White blood cell (WBC) counts and **d.**hemoglobin levels in peripheral blood after primary transplant. BM recovered from primary transplants was assayed for **e.**relative frequency of progenitors by FACS (± STD) **f.**colony forming units assay (CFU), and **g.**secondary competitive transplantation into wildtype recipients.
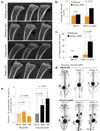
Figure 3. Ablation of the hematopoietic compartment in fatless A-ZIP/F1 mice during BM transplantation induces osteogenesis
Analysis of mice transplanted as in figure 2. **a.**High-resolution microCT analysis of pre/post-transplant tibias from wildtype (top) or fatless A-ZIP/F1 mice 20 days after lethal ablation. **b.**Average trabecular bone density of (a) normalized to a density standard (phantom). **c.**Percentage BM space occupied by trabecular bone 20 days after transplantation. **d.**MicroPET analysis pre/post-transplant. Representative mice shown at three different timepoints (3–4 analyzed per group). Dark areas indicate NaF-18 uptake in regions of active bone deposition (red arrowheads). **e.**Quantification of mean NaF-18 uptake in tibiae and proximal tails pre/post-transplantation. Square and lines over micrographs a. and d. indicate quantification regions (see methods).
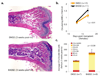
Figure 4. Pharmacological inhibition of adipocyte formation enhances BM engraftment in wild-type mice
BM transplants were performed in wild-type female FVB mice as described for figure 2 except that 30mg/kg BADGE or control vehicle (DMSO 10%) were administered through daily intra-peritoneal injections from the day prior to irradiation until day 14 post-transplant. **a.**H&E stain of femurs from mice sacrificed on day 17 post-transplant, when the donor CD45.2 wildtype BM was recovered and purified by FACS. **b.**White blood cell (WBC) counts in peripheral blood on the post-transplant period show accelerated recovery in BADGE-treated mice. **c.**Colony forming unit assay (CFU) from the recovered donor BM
Similar articles
- Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis.
Zhu RJ, Wu MQ, Li ZJ, Zhang Y, Liu KY. Zhu RJ, et al. Int J Hematol. 2013 Jan;97(1):58-72. doi: 10.1007/s12185-012-1233-4. Epub 2012 Dec 25. Int J Hematol. 2013. PMID: 23264188 - Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF.
Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ. Zhou BO, et al. Nat Cell Biol. 2017 Aug;19(8):891-903. doi: 10.1038/ncb3570. Epub 2017 Jul 17. Nat Cell Biol. 2017. PMID: 28714970 Free PMC article. - Lack of Adipocytes Alters Hematopoiesis in Lipodystrophic Mice.
Wilson A, Fu H, Schiffrin M, Winkler C, Koufany M, Jouzeau JY, Bonnet N, Gilardi F, Renevey F, Luther SA, Moulin D, Desvergne B. Wilson A, et al. Front Immunol. 2018 Nov 13;9:2573. doi: 10.3389/fimmu.2018.02573. eCollection 2018. Front Immunol. 2018. PMID: 30483254 Free PMC article. - Defining osteoblast and adipocyte lineages in the bone marrow.
Pierce JL, Begun DL, Westendorf JJ, McGee-Lawrence ME. Pierce JL, et al. Bone. 2019 Jan;118:2-7. doi: 10.1016/j.bone.2018.05.019. Epub 2018 May 18. Bone. 2019. PMID: 29782940 Free PMC article. Review. - The function of adipocytes in the bone marrow stroma: an update.
Gimble JM, Robinson CE, Wu X, Kelly KA. Gimble JM, et al. Bone. 1996 Nov;19(5):421-8. doi: 10.1016/s8756-3282(96)00258-x. Bone. 1996. PMID: 8922639 Review.
Cited by
- Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model.
Hazra S, Jarajapu YP, Stepps V, Caballero S, Thinschmidt JS, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern TS, Segal MS, Ash JD, Saban DR, Bartelmez SH, Grant MB. Hazra S, et al. Diabetologia. 2013 Mar;56(3):644-53. doi: 10.1007/s00125-012-2781-0. Epub 2012 Nov 29. Diabetologia. 2013. PMID: 23192694 Free PMC article. - How aging influences the gut-bone marrow axis and alters hematopoietic stem cell regulation.
Wells C, Robertson T, Sheth P, Abraham S. Wells C, et al. Heliyon. 2024 Jun 11;10(12):e32831. doi: 10.1016/j.heliyon.2024.e32831. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38984298 Free PMC article. Review. - Hematopoietic Stem Cell and Its Bone Marrow Niche.
Yu VW, Scadden DT. Yu VW, et al. Curr Top Dev Biol. 2016;118:21-44. doi: 10.1016/bs.ctdb.2016.01.009. Epub 2016 Mar 21. Curr Top Dev Biol. 2016. PMID: 27137653 Free PMC article. Review. - Differential Blood Counts Do Not Consistently Predict Clinical Measurements of Bone Mineral Density and Microarchitecture at Homeostasis.
Schyrr F, Marques-Vidal P, Hans D, Lamy O, Naveiras O. Schyrr F, et al. JBMR Plus. 2022 Aug 30;6(9):e10669. doi: 10.1002/jbm4.10669. eCollection 2022 Sep. JBMR Plus. 2022. PMID: 36111204 Free PMC article. - Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone.
Morris EV, Edwards CM. Morris EV, et al. Front Endocrinol (Lausanne). 2016 Jul 14;7:90. doi: 10.3389/fendo.2016.00090. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27471491 Free PMC article. Review.
References
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
- Zhang J, Niu C, Ye L, Huang H, He X, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. - PubMed
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. - PubMed
- Neumann E. Das Gesetz Verbreitung des gelben und rotten Markes in den Extremitätenknochen. Centralblatt für die medicinischen Wissenschaften. 1882;18:321–323.
- Calvo W, Fliedner TM, Herbst E, Hügl E, Bruch C. Regeneration of blood-forming organs after autologous leukocyte transfusion in lethally irradiated dogs. II. Distribution and cellularity of the marrow in irradiated and transfused animals. Blood. 1976;47:593–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK059279/DK/NIDDK NIH HHS/United States
- R01 DK070055/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- DP1 OD000256/OD/NIH HHS/United States
- T32- HL -7623/HL/NHLBI NIH HHS/United States
- DP1 OD000256-01/OD/NIH HHS/United States
- R01 DK070055-01/DK/NIDDK NIH HHS/United States
- R01 DK059279-06/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases