Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis - PubMed (original) (raw)
Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis
Elke Bayha et al. PLoS One. 2009.
Abstract
Background: Endoderm organ primordia become specified between gastrulation and gut tube folding in Amniotes. Although the requirement for RA signaling for the development of a few individual endoderm organs has been established a systematic assessment of its activity along the entire antero-posterior axis has not been performed in this germ layer.
Methodology/principal findings: RA is synthesized from gastrulation to somitogenesis in the mesoderm that is close to the developing gut tube. In the branchial arch region specific levels of RA signaling control organ boundaries. The most anterior endoderm forming the thyroid gland is specified in the absence of RA signaling. Increasing RA in anterior branchial arches results in thyroid primordium repression and the induction of more posterior markers such as branchial arch Hox genes. Conversely reducing RA signaling shifts Hox genes posteriorly in endoderm. These results imply that RA acts as a caudalizing factor in a graded manner in pharyngeal endoderm. Posterior foregut and midgut organ primordia also require RA, but exposing endoderm to additional RA is not sufficient to expand these primordia anteriorly. We show that in chick, in contrast to non-Amniotes, RA signaling is not only necessary during gastrulation, but also throughout gut tube folding during somitogenesis. Our results show that the induction of CdxA, a midgut marker, and pancreas induction require direct RA signaling in endoderm. Moreover, communication between CdxA(+) cells is necessary to maintain CdxA expression, therefore synchronizing the cells of the midgut primordium. We further show that the RA pathway acts synergistically with FGF4 in endoderm patterning rather than mediating FGF4 activity.
Conclusions/significance: Our work establishes that retinoic acid (RA) signaling coordinates the position of different endoderm organs along the antero-posterior axis in chick embryos and could serve as a basis for the differentiation of specific endodermal organs from ES cells.
Conflict of interest statement
Competing Interests: P. Serup and M. C. Jørgensen are employees and shareholders of Novo Nordisk A/S (Denmark).
Figures
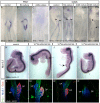
Figure 1. Endogenous RA signaling can be activated in endoderm.
Whole mount in situ hybridization analysis of Raldh2 (A–D), Cyp26A1 (E–H), RARα (I–M), RARβ (N–R) and RARγ (S–W) at gastrulation or early somitogenesis. Exact stages are indicated on whole mount pictures, ventral views, anterior to the top. 15 µm cryo-sections are shown in (C,D,G,H,K–M,P–R,U–W), dorsal side to the top. Red arrowheads point to expression in endoderm, blue arrowheads indicate cells within the PS, green arrowheads show the epiblast andwhite arrowheads point to Raldh2 expression in somites and LPM. Black lines in the whole mounts indicate the relative plane of sections. Scale bars are 100 µM. (X) RT-PCR analysis of RAR expression in endoderm versus mesoderm, which were harvested at stage HH 10. The integrated density of resulting bands were measured and normalized to tubulin expression. Each sample was made in duplets and bars show the mean. Error bars indicate standard deviation. AE, axial endoderm; LPE, lateral plate endoderm; LPM, lateral plate mesoderm; SM, somitic mesoderm.
Figure 2. Exogenous RA activates ectopic gene transcription in the endoderm.
(A) is a schematic illustration showing the initial grafting position of the RA bead in stage HH 4− or HH 10 chick embryos. (B–I) show whole mount in situ hybridization analysis of Cyp26A1 in control (B,F), RA bead grafted (C,G) or inhibitor treated embryos (D,H), ventral view, anterior to the top. Beads were soaked in ethanol (control) or ethanol containing 10−3 M RA and were grafted onto the endoderm and analyzed 6 hours later (approximately HH 5 or HH 11, respectively). For inhibition, embryos were treated with 10−5 M AGN193109 added to the culture medium at HH 3+ or HH 10, incubated 6 hours and then analyzed (approximately HH 4–5 or HH 11, respectively). Exact stage of grafting and analysis are given in each picture. Position of beads is marked by a circle and lines give the plane of sections shown in (E and I), ventral side down. Scale bars are 100 µM. Note broad induction in (E) and unilateral induction ventrally in (I). A, anterior; h, hours; P, posterior.
Figure 3. RA patterns pharyngeal endoderm in graded manner.
RA beads (10−3 M) and control beads were grafted at HH 10 onto endoderm and embryos were analyzed at different stages corresponding to the time when the specific marker is expressed (D,E,G,H,J,K,M,N). Hex expression was also analyzed in embryos grafted at HH 4 and analyzed at HH 5 (A,B). Inhibition of RA was conducted in stage HH 3+ or HH 4 chick embryos by adding 10−5 M AGN193109 to the culture medium (C,F,I,L,O). Exact stages of treatment and analysis are indicated in each picture. Whole mount in situ hybridization analyzed embryos for Hex (A–F), Nkx2.1 (G–I), Prox1 (J–L) and HoxB4 (M–O). In lateral views of Nkx2.1 (G–I), surface ectoderm was removed to see better the staining. Box in (K) shows higher magnification of Prox1 inhibition by RA treatment. Although liver Hex expression may appear reduced in E, this is not reproducibly observed. The black line marks the midline at the AIP. HoxB4 is expressed in all three germ layers, therefore, induction in endoderm has been verified by 15 µm sections (P,Q) marked by black lines in the corresponding whole mount embryos. Anterior is always at the top except in (G–I) where anterior is shown to the left. Scale bars are 100 µM. Asterisks mark the otic vesicle. Black arrowheads mark the loss of thyroid gene expression in RA treated embryos. Black arrows point to loss of Hex or Prox1 expression. Blue arrowheads indicate normal thyroid expression of Hex or Nkx2.1. Green arrowheads point to the liver bud. Red arrowhead shows ectopic Hex expression. White arrowhead marks forebrain expression of Nkx2.1. Circles mark the position of grafted beads.
Figure 4. RA is essential to pattern the posterior foregut and midgut domains.
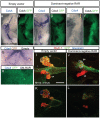
Embryos are treated either with 10−6 M RA in the culture medium at HH 10 (B, E) or with 10−5 M AGN193109 in the culture medium at stage HH 4 (C,F,G) or HH 8 (D,E). Exact stages of treatment and analysis are indicated in each picture. Anterior is always to the top. Ventral view of whole mount in situ hybridized embryos for expression of Pdx1 (A–C) and CdxA (D–G). RA activation had no effect on Pdx1 or CdxA (B, E). Inhibition of RA resulted in complete inhibition of Pdx1 (C) and two different phenotypes for CdxA. Either its expression was completely inhibited (F, 63%) or shifted towards posterior (G, 37%). Black arrowheads point to the AIP. Black arrows show the anterior boundary of CdxA expression. Note that the embryo in F has not formed the AIP properly. Side view of whole mount in situ hybridization for Nkx6.2 after exposure to indicated amounts of AGN193109 in the culture medium from stage HH8 (4 somites) to HH16–17 (H–K). Nkx6.2 expression in the pancreas is progressively reduced while expression in the nervous system is unaffected. Subsequent organogenesis was assessed at stage HH 19 by whole mount immunostaining for Pdx1 (pancreas and duodenum, green), Nkx6.1 (pancreas and subset of duodenum, blue) and glucagon (alpha cells, red) after exposure to indicated amounts of AGN193109 (L–O). Anterior is always to the top. (DP) Dorsal pancreas (VP) Ventral pancreas.
Figure 5. Electroporation of dominant negative RARs abolishes CdxA expression and pancreas formation.
Electroporation of pCIG (A,B,G,I,K) or pCIG-DNRAR (C–F,H,J,L). Whole mount in situ hybridization on stage HH 19 embryos shows CdxA expression (blue) in the closed duodenum and the open midgut. CdxA expression is repressed either partially (n = 7/11, C) or completely (n = 3/11, F) by DN-RAR. Cells expressing the expression construct are labeled by subsequent immunocytochemistry for GFP expressed from the bicistronic construct (Green in D, F and H, masked by blue CdxA staining in B, DAB-brown in G), demonstrating that repression extends to the neighbors of targeted cells. Whole mount immunocytochemistry on stage HH 20 embryos shows that dominant negative RAR (traced with GFP, green) represses pancreas progenitor emergence (traced with Nkx6.1, red) in a non-cell autonomous manner (J) as compared to control embryos electroporated with empty vector (I). Glucagon+ cells could still differentiate (blue). (K and L) Selected optical sections of embryos displayed in (I) and (J), respectively. Scale bar 200 µm.
Figure 6. RA and FGF4 independently pattern the anterior endoderm.
Whole mount in situ hybridization analysis of Hex expression. Ventral view, anterior to the top. (A–D) Analysis of embryos when FGF4 signaling is activated and RA signaling is inhibited. Embryos were treated at stage HH 3+ with DMSO and grafted with PBS beads (A) as control, treated with DMSO and grafted with FGF4 beads (1 mg/ml) (B), treated with10−5 M AGN193109 and grafted with PBS beads (C), or treated with 10−5 M AGN193109 and grafted with FGF4 beads (1 mg/ml) (D). Circles show position of beads in (A–D). (E–H) Analysis of embryos when FGF4 signaling is inhibited and RA signaling is activated. Embryos were treated at stage HH 3+ either with DMSO and ethanol (E) as control, with DMSO and 10−6 M RA (F), with 20 µM SU5402 and ethanol (G), or with 10−6 M RA and 20 µM SU5402 (H).Treatment was done at stage HH3+ and 24 embryos were analyzed 6 hours later at stage HH 4–5. Exact stages of treatment and analysis are indicated in each picture. (I) FGF4 activity is independent of RA. Embryos were treated and analyzed as (E–H). The “length of Hex domain/length of embryo” ratio in % was calculated (DMSO/ethanol n = 10, DMSO/RA n = 8, SU5402/ethanol n = 9, SU5402/RA n = 6). Bars in the diagram represent the mean and error bars display the standard error of the mean. The P-value was less than 0.001 (Student t test) between control and RA treated embryos and between RA treated and RA/SU5402 treated embryos (two asterisks). The P-value was less than 0.05 (Student t test) between control and SU5402 treated embryos (one asterisk). RA/SU5402 showed no significant difference from control embryos.
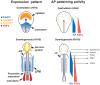
Figure 7. Model for AP patterning of RA and FGFs in endoderm.
At gastrulation and early somitogenesis RA is synthesized in the posterior part of the embryo with its anterior limit around the junction between prospective fore- and hindgut. RA degrading enzyme Cyp26A1 is expressed anteriorly and functions as intracellular sink for RA molecules (upper panel to the left). A RA gradient may be formed in the intermediate region. At the same time FGFs are expressed in the node and in the posterior streak acting in a graded manner along the entire AP axis (upper panel to the right). At stage HH 10 graded activation of RA signaling may be maintained in the dorsal (axial) endoderm anterior to the 6th somite and in the foregut, while LPE is constantly exposured to RA (lower panel). FGFs are produced caudally in the tail bud (lower panel to the left), again acting in a graded manner along the AP axis. Posteriorly, RA may form a contra-gradient to FGFs antagonizing each other as it was shown in pre-somitic mesoderm. Different levels of signaling gradients induce different gene transcription. Whether FGFs also induce genes at the level of the posterior BAs, is not known (lower panel to the right). Color code is explained in the legend beside. Dotted orange lines represent presumptive neural plate. LPE, lateral plate endoderm; LPM, lateral plate mesoderm.
Similar articles
- FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo.
Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. Dessimoz J, et al. Mech Dev. 2006 Jan;123(1):42-55. doi: 10.1016/j.mod.2005.10.001. Epub 2005 Dec 2. Mech Dev. 2006. PMID: 16326079 - FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner.
Johannesson M, Ståhlberg A, Ameri J, Sand FW, Norrman K, Semb H. Johannesson M, et al. PLoS One. 2009;4(3):e4794. doi: 10.1371/journal.pone.0004794. Epub 2009 Mar 11. PLoS One. 2009. PMID: 19277121 Free PMC article. - Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification.
Kopinke D, Sasine J, Swift J, Stephens WZ, Piotrowski T. Kopinke D, et al. Dev Dyn. 2006 Oct;235(10):2695-709. doi: 10.1002/dvdy.20905. Dev Dyn. 2006. PMID: 16871626 - Movements of chick gastrulation.
Voiculescu O. Voiculescu O. Curr Top Dev Biol. 2020;136:409-428. doi: 10.1016/bs.ctdb.2019.11.015. Epub 2019 Dec 26. Curr Top Dev Biol. 2020. PMID: 31959297 Review. - Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin's PhD thesis.
Grapin-Botton A. Grapin-Botton A. Int J Dev Biol. 2005;49(2-3):335-47. doi: 10.1387/ijdb.041946ag. Int J Dev Biol. 2005. PMID: 15906249 Review.
Cited by
- TGF-β modulates cell fate in human ES cell-derived foregut endoderm by inhibiting Wnt and BMP signaling.
Funa NS, Mjoseng HK, de Lichtenberg KH, Raineri S, Esen D, Egeskov-Madsen AR, Quaranta R, Jørgensen MC, Hansen MS, van Cuyl Kuylenstierna J, Jensen KB, Miao Y, Garcia KC, Seymour PA, Serup P. Funa NS, et al. Stem Cell Reports. 2024 Jul 9;19(7):973-992. doi: 10.1016/j.stemcr.2024.05.010. Epub 2024 Jun 27. Stem Cell Reports. 2024. PMID: 38942030 Free PMC article. - Reciprocal Interactions Between the Epithelium and Mesenchyme in Organogenesis.
Kondoh H. Kondoh H. Results Probl Cell Differ. 2024;72:119-126. doi: 10.1007/978-3-031-39027-2_7. Results Probl Cell Differ. 2024. PMID: 38509255 - A chemo-mechanical model of endoderm movements driving elongation of the amniote hindgut.
Oikonomou P, Cirne HC, Nerurkar NL. Oikonomou P, et al. bioRxiv [Preprint]. 2023 May 18:2023.05.18.541363. doi: 10.1101/2023.05.18.541363. bioRxiv. 2023. PMID: 37292966 Free PMC article. Updated. Preprint. - Effects of all-trans and 9-cis retinoic acid on differentiating human neural stem cells in vitro.
Kubickova B, Martinkova S, Bohaciakova D, Nezvedova M, Liu R, Brozman O, Spáčil Z, Hilscherova K. Kubickova B, et al. Toxicology. 2023 Mar 15;487:153461. doi: 10.1016/j.tox.2023.153461. Epub 2023 Feb 16. Toxicology. 2023. PMID: 36805303 Free PMC article. - Retinoic Acid Promotes the In Vitro Growth, Patterning and Improves the Cellular Composition of Human Pluripotent Stem-Cell-Derived Intestinal Organoids.
Qu N, Jeffcoat B, Maity P, Christensen RK, Múnera JO. Qu N, et al. Int J Mol Sci. 2022 Aug 3;23(15):8624. doi: 10.3390/ijms23158624. Int J Mol Sci. 2022. PMID: 35955755 Free PMC article.
References
- Yasugi S. Role of Epithelial-Mesenchymal Interactions in Differentiation of Epithelium of Vertebrate Digestive Organs. DevelopGrowth&Differ. 1993;35:1–9. - PubMed
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. - PubMed
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. - PubMed
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources