Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring - PubMed (original) (raw)
Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring
Shona L Kirk et al. PLoS One. 2009.
Abstract
Hypothalamic systems which regulate appetite may be permanently modified during early development. We have previously reported hyperphagia and increased adiposity in the adult offspring of rodents fed an obesogenic diet prior to and throughout pregnancy and lactation. We now report that offspring of obese (OffOb) rats display an amplified and prolonged neonatal leptin surge, which is accompanied by elevated leptin mRNA expression in their abdominal white adipose tissue. At postnatal Day 30, before the onset of hyperphagia in these animals, serum leptin is normal, but leptin-induced appetite suppression and phosphorylation of STAT3 in the arcuate nucleus (ARC) are attenuated; the level of AgRP-immunoreactivity in the hypothalamic paraventricular nucleus (PVH), which derives from neurones in the ARC and is developmentally dependent on leptin, is also diminished. We hypothesise that prolonged release of abnormally high levels of leptin by neonatal OffOb rats leads to leptin resistance and permanently affects hypothalamic functions involving the ARC and PVH. Such effects may underlie the developmental programming of hyperphagia and obesity in these rats.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
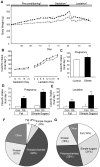
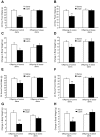
Figure 1. Maternal body weight and food intake in rats fed control or obesogenic diet.
Body weight (A) was recorded for 6 weeks prior to pregnancy and throughout pregnancy and lactation for the animals on the control (open symbols) or obesogenic (closed symbols) diet; calorific intake was recorded throughout pregnancy and lactation (B). Average daily calorific intake from all sources during pregnancy (C) and average daily calorific intake from fat or simple sugars during pregnancy (D) or lactation (E) for the animals on the control (Con) or obesogenic (Ob) diet. Macronutrient content of ingested food (expressed as percentage by weight) for control (F) or obese (G) dams during lactation; “other” includes cellulose, ash, water etc. * p<0.05 and ** p<0.01 versus control dams (n = 11–12).
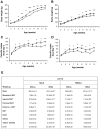
Figure 2. Post-weaning characteristics of offspring of control and obese dams.
Body weight for males (A) and females (B) and calorific intake for males (C) and females (D) were recorded post-weaning for offspring of control (open symbols) or obese (closed symbols) dams. Offspring body and tissue weights were recorded at postnatal Day 90 (E). OffCon = offspring of control dams; OffOb = offspring of obese dams; WAT = white adipose tissue; BAT = brown adipose tissue; s.c. = subcutaneous. * p<0.05 and ** p<0.01 versus offspring of control dams (n = 8–11).
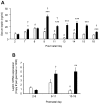
Figure 3. Neonatal serum leptin concentrations and adipose leptin mRNA expression in offspring of control and obese dams.
Serum leptin was measured in offspring of control dams (open bars) and obese dams (closed bars) on postnatal days 2, 7, 8, 9, 11, 13, 14, 15 and 18 (A). Leptin mRNA expression in abdominal fat is presented for postnatal days 2–8, 9–11 and 13–18 (B). * p<0.05, ** p<0.01 and *** p<0.01 versus offspring of control dams for the same period (n = 3–6). For longitudinal comparisons, a significant difference (p<0.05) from the preceding period is indicated by # for offspring of control dams and by † for offspring of obese dams.
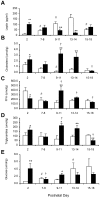
Figure 4. Constituents of milk from control and obese dams by analysis of pups' stomach contents.
The concentration of leptin (A), cholesterol (B), free fatty acids (FFA) (C), triglycerides (D) and glucose (E) was assayed in stomach contents (as a proxy measure of the dams' milk content) from offspring of control dams (open bars; n = 4–8) and obese dams (closed bars; n = 4–8) throughout suckling. * p<0.05 and ** p<0.01 versus offspring of control dams at the same period. For longitudinal comparisons, a significant difference (p<0.05) from the preceding period is indicated by # for offspring of control dams and by † for offspring of obese dams.
Figure 5. Behavioural responses to leptin in juvenile and adult offspring of control and obese dams.
Food intake for males (A) and females (B) and change in body weight for males (C) and females (D) recorded over 24 hours following administration of leptin (10 mg/kg, i.p.) in 30 day-old offspring of control or obese dams. Food intake for males (E) and females (F) and change in body weight for males (G) and females (H) in 90 day-old offspring of control or obese dams. OffCon = offspring of control dams; OffOb = offspring of obese dams; * p<0.05 and ** p<0.01 ***p<0.001, versus offspring of control dams (n = 6).
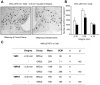
Figure 6. Signalling responses to leptin in juvenile and adult offspring of control and obese dams.
Representative images (A) and quantitative analysis (B and C) of leptin-induced pSTAT3-immunoreactive (ir) cells (per mm2) in the arcuate nucleus (ARC) and ventromedial hypothalamic nucleus (VMH). Leptin (10 mg/kg, i.p.) was administered 45 min prior to administration of anaesthetic and perfusion fixation of the brain. OffCon = offspring of control dams; OffOb = offspring of obese dams; dl = dorsolateral; vl = ventrolateral. * p<0.05 versus offspring of control dams (n = 4–8).
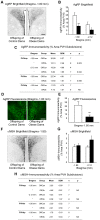
Figure 7. AgRP- and α-MSH-immunoreactivity in the PVH of offspring of control and obese dams.
Representative brightfield images (A) and quantitative comparisons (B,C) of AgRP-immunoreactivity in the paraventricular hypothalamic nucleus (PVH) and its subdivisions in male offspring of control and obese dams. Representative confocal images (D) and quantitative comparisons (D,E) of AgRP immunofluorescence in the PVH of female offspring of control and obese dams. Representative brightfield images (F) and quantitative comparisons (G,H) of α-MSH-immunoreactivity in the PVH and its subdivisions in male offspring of control and obese dams. OffCon = offspring of control dams (open bars); OffOb = offspring of obese dams (closed bars); mp = medial parvocellular; lm = lateral magnocellular; dp = dorsal parvocellular. * = p<0.05 and ** = p<0.01 versus control. Scale bars = 200 µm.
Similar articles
- Gestational hypoxia disrupts the neonatal leptin surge and programs hyperphagia and obesity in male offspring in the Sprague-Dawley rat.
Vargas VE, Gurung S, Grant B, Hyatt K, Singleton K, Myers SM, Saunders D, Njoku C, Towner R, Myers DA. Vargas VE, et al. PLoS One. 2017 Sep 28;12(9):e0185272. doi: 10.1371/journal.pone.0185272. eCollection 2017. PLoS One. 2017. PMID: 28957383 Free PMC article. - Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats.
Almeida MM, Dias-Rocha CP, Reis-Gomes CF, Wang H, Atella GC, Cordeiro A, Pazos-Moura CC, Joss-Moore L, Trevenzoli IH. Almeida MM, et al. Psychoneuroendocrinology. 2019 May;103:306-315. doi: 10.1016/j.psyneuen.2019.02.004. Epub 2019 Feb 8. Psychoneuroendocrinology. 2019. PMID: 30776574 - The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box.
Williams G, Harrold JA, Cutler DJ. Williams G, et al. Proc Nutr Soc. 2000 Aug;59(3):385-96. doi: 10.1017/s0029665100000434. Proc Nutr Soc. 2000. PMID: 10997654 Review. - The Role of PVH Circuits in Leptin Action and Energy Balance.
Sutton AK, Myers MG Jr, Olson DP. Sutton AK, et al. Annu Rev Physiol. 2016;78:207-21. doi: 10.1146/annurev-physiol-021115-105347. Annu Rev Physiol. 2016. PMID: 26863324 Free PMC article. Review.
Cited by
- Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort.
Vollbach H, Brandt S, Lahr G, Denzer C, von Schnurbein J, Debatin KM, Wabitsch M. Vollbach H, et al. Int J Obes (Lond). 2017 Jan;41(1):13-22. doi: 10.1038/ijo.2016.161. Epub 2016 Sep 22. Int J Obes (Lond). 2017. PMID: 27654141 - Early life overnutrition impairs plasticity of non-neuronal brainstem cells and drives obesity in offspring across development in rats.
Liberini CG, Ghidewon M, Ling T, Lhamo R, Juntereal N, Stein LM, Hayes MR. Liberini CG, et al. Int J Obes (Lond). 2020 Dec;44(12):2405-2418. doi: 10.1038/s41366-020-00658-5. Epub 2020 Sep 30. Int J Obes (Lond). 2020. PMID: 32999409 - Thyroid Hormones and Cortisol Concentrations in Offspring are Influenced by Maternal Supranutritional Selenium and Nutritional Plane in Sheep.
Vonnahme KA, Neville TL, Lekatz LA, Reynolds LP, Hammer CJ, Redmer DA, Caton JS. Vonnahme KA, et al. Nutr Metab Insights. 2013 Apr 17;6:11-21. doi: 10.4137/NMI.S11332. Print 2013. Nutr Metab Insights. 2013. PMID: 23935368 Free PMC article. - Early overnutrition alters synaptic signaling and induces leptin resistance in arcuate proopiomelanocortin neurons.
Roberts BL, Bennett CM, Carroll JM, Lindsley SR, Kievit P. Roberts BL, et al. Physiol Behav. 2019 Jul 1;206:166-174. doi: 10.1016/j.physbeh.2019.04.002. Epub 2019 Apr 2. Physiol Behav. 2019. PMID: 30951750 Free PMC article. - Feed your head: neurodevelopmental control of feeding and metabolism.
Lee DA, Blackshaw S. Lee DA, et al. Annu Rev Physiol. 2014;76:197-223. doi: 10.1146/annurev-physiol-021113-170347. Epub 2013 Nov 18. Annu Rev Physiol. 2014. PMID: 24274739 Free PMC article. Review.
References
- Catalano PM. Obesity and pregnancy–the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–3506. - PubMed
- Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:1674S–1683S. - PubMed
- Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. AmJObstetGynecol. 2003;189:1698–1704. - PubMed
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous