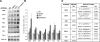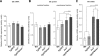Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice - PubMed (original) (raw)
. 2009 Aug 14;386(1):257-62.
doi: 10.1016/j.bbrc.2009.06.032. Epub 2009 Jun 10.
Georgie Scales, Karelle Leroy, Mirsada Causevic, Claudie Hooper, Elaine E Irvine, Agharul I Choudhury, Laura Drinkwater, Fiona Kerr, Hind Al-Qassab, John Stephenson, Zehra Yilmaz, K Peter Giese, Jean-Pierre Brion, Dominic J Withers, Simon Lovestone
Affiliations
- PMID: 19523444
- PMCID: PMC2726921
- DOI: 10.1016/j.bbrc.2009.06.032
Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice
Richard Killick et al. Biochem Biophys Res Commun. 2009.
Abstract
As impaired insulin signalling (IIS) is a risk factor for Alzheimer's disease we crossed mice (Tg2576) over-expressing human amyloid precursor protein (APP), with insulin receptor substrate 2 null (Irs2(-/-)) mice which develop insulin resistance. The resulting Tg2576/Irs2(-/-) animals had increased tau phosphorylation but a paradoxical amelioration of Abeta pathology. An increase of the Abeta binding protein transthyretin suggests that increased clearance of Abeta underlies the reduction in plaques. Increased tau phosphorylation correlated with reduced tau-phosphatase PP2A, despite an inhibition of the tau-kinase glycogen synthase kinase-3. Our findings demonstrate that disruption of IIS in Tg2576 mice has divergent effects on pathological processes-a reduction in aggregated Abeta but an increase in tau phosphorylation. However, as these effects are accompanied by improvement in behavioural deficits, our findings suggest a novel protective effect of disrupting IRS2 signalling in AD which may be a useful therapeutic strategy for this condition.
Figures
Fig. 1
Phospho-tau immunoreactivity is increased in temporal cortex of Tg2576/Irs2−/− mice. (A) Phosphospecific anti-tau antibodies were used to probe temporal cortex of age-matched wild-type (WT); Tg2576; Irs2_−/_− and Tg2576/Irs2−/− animals. (B) Data shown is normalised to a phosphorylation-independent tau antibody. (C) Analysis by genotype (ANOVA with post hoc Tukeys test; n = 29) showed significant increases in tau phosphorylation in Tg2576/Irs2−/− animals relative to WT and Tg2576 animals at epitopes recognised by antibodies MC6, CP13, PG5, PHF1, and TG3 but not at the overlapping AT8 and TAU1 epitopes.
Fig. 2
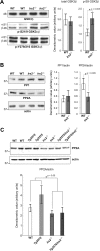
GSK-3 is inhibited and PP2a is reduced in mice lacking Irs2. The tau-kinase, GSK-3 and the protein phosphatases PP1 and PP2A were examined in wild-type (WT) and in IRS2−/− mice by Western blot. (A) Animals lacking Irs2 showed no change in total GSK-3 protein or in phosphorylation at the Tyr279 in GSK-3α/GSK-3β 216 epitope. However there was a substantial (p < 0.05) increase in GSK-3 phosphorylation at Ser 21 GSK-3α/Ser 9 GSK-3β epitope reflecting relative inhibition of GSK-3 activity in these animals. (B) There was no change in PP1 but a significant increase in PP2A in animals lacking Irs2. (C) Comparing PP2a in hippocampus across all four genotypes confirmed a reduction in PP2a in all animals lacking Irs2 but no effect of APP expression.
Fig. 3
Deletion of Irs2 reduces Aβ deposition and decreases insoluble A_β. (A) Immunocytochemical labeling with a human specific anti-Aβ antibody of whole brain frontal cortex sections of 12 month-old Tg2576 and Tg2576/Irs2−/− mice. Scale bar = 200 microns. (B) Immunocytochemical with the anti-Aβ antibody (left panels) and histochemical labeling with Congo red (right panels) of temporal cortex sections of 12 month-old Tg2576 and Tg2576/Irs2−/− mice, showing the extent of Aβ deposition in representative animals. Scale bars = 50 microns. (C) The mean surface covered by Aβ deposits in the cortex was significantly reduced in Tg2576/Irs2−/− mice compared with Tg2576 mice (n = 32; ∗_p = 0.01, t_-test). (D) APP processing was examined by immunoblotting for holo-APP and APP-CTFs in temporal cortex of Tg2576 and Tg2576/Irs2−/− animals. Holo-APP values were normlised to actin, CTF values were normalised to holo-APP. No differences were found between genotypes. (E) Human Aβ1–40 and Aβ1–42 were measured by ELISA in S1 (soluble) and S2 (detergent soluble) fractions and formic acid extracts of P2 pellets from temporal cortex. Significant reductions in Aβ1–40 and Aβ1–42 were found in formic acid extracts from Tg2576/Irs2−/− compared to Tg2576 animals (n = 21; ∗_p = 0.0005 [Aβ1–40] and 0.002 [Aβ1–42], _t_-test).
Fig. 4
Increase in insulin degrading enzyme and in transthyretin in mice lacking Irs2. Amyloid turnover is regulated by proteolysis through proteases such as insulin degrading enzyme (IDE) and by increased clearance following binding to proteins such as transthyretin; both known to be altered in response to insulin signalling. IDE mRNA was unaltered in IRS2−/− mice (A) but protein levels in the membrane-bound fraction were modestly increased (B). Transthyretin mRNA was increased substantially (3.9-fold; p < 0.02; SEM 1.05) in both genotypes lacking Irs2.
Similar articles
- FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer's disease via decreasing beta-amyloid production and tau hyperphosphorylation.
Bao XQ, Li N, Wang T, Kong XC, Tai WJ, Sun H, Zhang D. Bao XQ, et al. PLoS One. 2013 Nov 4;8(11):e78033. doi: 10.1371/journal.pone.0078033. eCollection 2013. PLoS One. 2013. PMID: 24223757 Free PMC article. - Differential involvement of insulin receptor substrate (IRS)-1 and IRS-2 in brain insulin signaling is associated with the effects on amyloid pathology in a mouse model of Alzheimer's disease.
Ochiai T, Sano T, Nagayama T, Kubota N, Kadowaki T, Wakabayashi T, Iwatsubo T. Ochiai T, et al. Neurobiol Dis. 2021 Nov;159:105510. doi: 10.1016/j.nbd.2021.105510. Epub 2021 Sep 16. Neurobiol Dis. 2021. PMID: 34537327 - Insulin receptor signaling mediates APP processing and β-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer's disease.
Stöhr O, Schilbach K, Moll L, Hettich MM, Freude S, Wunderlich FT, Ernst M, Zemva J, Brüning JC, Krone W, Udelhoven M, Schubert M. Stöhr O, et al. Age (Dordr). 2013 Feb;35(1):83-101. doi: 10.1007/s11357-011-9333-2. Epub 2011 Nov 6. Age (Dordr). 2013. PMID: 22057897 Free PMC article. - Xanthoceraside rescues learning and memory deficits through attenuating beta-amyloid deposition and tau hyperphosphorylation in APP mice.
Jin G, Wang LH, Ji XF, Chi TY, Qi Y, Jiao Q, Xu Q, Zhou XY, Zhang R, Zou LB. Jin G, et al. Neurosci Lett. 2014 Jun 24;573:58-63. doi: 10.1016/j.neulet.2014.04.032. Epub 2014 May 5. Neurosci Lett. 2014. PMID: 24810883 - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
- An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers.
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. Bomfim TR, et al. J Clin Invest. 2012 Apr;122(4):1339-53. doi: 10.1172/JCI57256. J Clin Invest. 2012. PMID: 22476196 Free PMC article. - Comorbidity Genes of Alzheimer's Disease and Type 2 Diabetes Associated with Memory and Cognitive Function.
Cho SB. Cho SB. Int J Mol Sci. 2024 Feb 12;25(4):2211. doi: 10.3390/ijms25042211. Int J Mol Sci. 2024. PMID: 38396891 Free PMC article. Review. - Endoplasmic reticulum stress impairs insulin receptor signaling in the brains of obese rats.
Liang L, Chen J, Zhan L, Lu X, Sun X, Sui H, Zheng L, Xiang H, Zhang F. Liang L, et al. PLoS One. 2015 May 15;10(5):e0126384. doi: 10.1371/journal.pone.0126384. eCollection 2015. PLoS One. 2015. PMID: 25978724 Free PMC article. - Integrating metabolism and longevity through insulin and IGF1 signaling.
Sadagurski M, White MF. Sadagurski M, et al. Endocrinol Metab Clin North Am. 2013 Mar;42(1):127-48. doi: 10.1016/j.ecl.2012.11.008. Epub 2012 Dec 21. Endocrinol Metab Clin North Am. 2013. PMID: 23391244 Free PMC article. Review. - Differential effects of delayed aging on phenotype and striatal pathology in a murine model of Huntington disease.
Tallaksen-Greene SJ, Sadagurski M, Zeng L, Mauch R, Perkins M, Banduseela VC, Lieberman AP, Miller RA, Paulson HL, Albin RL. Tallaksen-Greene SJ, et al. J Neurosci. 2014 Nov 19;34(47):15658-68. doi: 10.1523/JNEUROSCI.1830-14.2014. J Neurosci. 2014. PMID: 25411494 Free PMC article.
References
- Carro E., Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004;490:127–133. - PubMed
- Adlerz L., Holback S., Multhaup G., Iverfeldt K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J. Biol. Chem. 2007;282:10203–10209. - PubMed
- Solano D.C., Sironi M., Bonfini C., Solerte S.B., Govoni S., Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 2000;14:1015–1022. - PubMed
- Hong M., Lee V.M.Y. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 1997;272:19547–19553. - PubMed
- Lesort M., Johnson G.V.W. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials