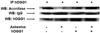Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis - PubMed (original) (raw)
Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis
V Panduri et al. Free Radic Biol Med. 2009.
Abstract
8-Oxoguanine DNA glycosylase (Ogg1) repairs 8-oxo-7,8-dihydroxyguanine (8-oxoG), one of the most abundant DNA adducts caused by oxidative stress. In the mitochondria, Ogg1 is thought to prevent activation of the intrinsic apoptotic pathway in response to oxidative stress by augmenting DNA repair. However, the predominance of the beta-Ogg1 isoform, which lacks 8-oxoG DNA glycosylase activity, suggests that mitochondrial Ogg1 functions in a role independent of DNA repair. We report here that overexpression of mitochondria-targeted human alpha-hOgg1 (mt-hOgg1) in human lung adenocarcinoma cells with some alveolar epithelial cell characteristics (A549 cells) prevents oxidant-induced mitochondrial dysfunction and apoptosis by preserving mitochondrial aconitase. Importantly, mitochondrial alpha-hOgg1 mutants lacking 8-oxoG DNA repair activity were as effective as wild-type mt-hOgg1 in preventing oxidant-induced caspase-9 activation, reductions in mitochondrial aconitase, and apoptosis, suggesting that the protective effects of mt-hOgg1 occur independent of DNA repair. Notably, wild-type and mutant mt-hOgg1 coprecipitate with mitochondrial aconitase. Furthermore, overexpression of mitochondrial aconitase abolishes oxidant-induced apoptosis whereas hOgg1 silencing using shRNA reduces mitochondrial aconitase and augments apoptosis. These findings suggest a novel mechanism that mt-hOgg1 acts as a mitochondrial aconitase chaperone protein to prevent oxidant-mediated mitochondrial dysfunction and apoptosis that might be important in the molecular events underlying oxidant-induced toxicity.
Figures
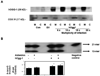
Figure 1. Mitochondria-targeted hOggl augments hOggl mitochondrial protein and 8-oxoG DNA glycosylase activity in A549 cells
(A) Mt-hOggl was transiently overexpressed using an adenovirus containing the α isoform of the hOGGl gene with a mitochondrial targeting sequence. A representative Western blot analysis of hOggl protein expression in mitochondrial (M) and cytosolic (C) fractions of control (con), empty vector (ev), and hOggl overexpression in A549 cells exposed to various multiplicity of infection (MOI) for 48 h. A MOI of lOx was used for all subsequent adenoviral transient transfection experiments. The Western blot is representative of three separate experiments. (B): Mitochondria-targeted hOggl augments 8-oxoG DNA glycosylase activity. A549 cells were transiently transfected with empty vector control (lanes 1 and 2) or mt-hOggl (lanes 3 and 4) as described in the Experimental Procedures and then treated with control media or asbestos (25 µg/cm2) for 24 h. 8-oxoG incision activity was calculated as described in the Experimental Procedures and expressed as fold control levels. (* p < .05 v. empty vector controls; n=3).
Figure 2. Mitochondria-targeted hOggl blocks oxidant-induced A549 cell mitochondrial dysfunction and apoptosis
(A) Mt-hOgg-1 overexpression prevents amosite asbestos-induced ΔΨm as assessed using a tetramethylrhodamine ethyl ester (TMRE) fluorometric technique. (B): Mt-hOggl overexpression abolishes asbestos-induced caspase-9 activation and DNA nucleosomal fragmentation.(C): Mt-hOggl overexpression blocks H2O2-induced ΔΨm (TMRE fluorescence) and DNA fragmentation. * p < .05 v. no asbestos or H2O2; † p < .05 v. empty vector cells exposed to the same dose of asbestos or H2O2.
Figure 3. Mitochondria-targeted hOggl does not alter oxidant-induced ROS production but does prevent reductions in mitochondrial aconitase
(A) Mt-hOggl overexpression does not alter amosite asbestos- or Antimycin A-induced ROS production as assessed by a dichlorofluorescein (DCF) assay. (B) Mt-hOggl overexpression does not block asbestos-induced ROS production as assessed by a roGFP sensor targeted to the mitochondria or cytosol. (C) As expected, mitochondrial aconitase activity is reduced by oxidative stress (24 h exposure to amosite asbestos or H2O2). Notably, mt-hOggl overexpression completely preserves mitochondrial aconitase activity in the setting of oxidative stress. (D) Amosite asbestos decreases mitochondrial aconitase protein expression and mt-hOggl overexpression blocks this. The levels of mitochondrial aconitase expression from three experiments are shown in a densitometric analysis. Expression of cytochrome oxidase IV (COX IV) was used to confirm the presence of mitochondrial protein and comparable loading. * p < .05 v. empty vector controls not exposed to H2O2, Antimycin A, or asbestos; † p < .05 v. empty vector cells exposed to the same dose of H2O2 or asbestos.
Figure 4. Mitochondrial hOggl co-precipitates with mitochondrial aconitase
Immunoprecipitation (IP) using a hOggl antibody was performed on mitochondria proteins obtained from control and mt-hOggl overexpressing A549 cells in the absence or presence of asbestos (25 µg/cm2) for 24 h as described in the Methods. Mitochondrial aconitase and loading controls (IgG and hOggl) were assessed by Western blotting (WB). The immunoprecipitation blots are representative of three independent experiments.
Figure 5. Mitochondrial hOggl prevents oxidant-induced toxicity independent of its 8-oxoG DNA repair activity
Transient overexpression of empty vector control, wild-type mt-hOgg-1 as well as two previously described hOgg-1 mutant plasmid constructs lacking 8-oxoguanine DNA repair capacity (317–323 long alpha/beta and V317) were done by lipofectamine as described in the Methods (18). Mitochondrial localization was confirmed by C-Myc immunostaining. Similar to wild-type mt-hOgg-1, overexpression of mt-hOgg-1 mutants 317–323 long alpha/beta or V317 completely blocked oxidant induced caspase-9 activation (A), DNA fragmentation (B), and the decrease in mitochondrial aconitase activity (C) caused by H2O2 (100 µM) or asbestos (25 µg/cm2) for 24h. * p < .05 v. no H2O2 or asbestos; † p < .05 v. empty vector cells exposed to the same dose of 2O2 or asbestos.
Figure 6. Mitochondrial wild-type and long alpha/beta 317–323 hOggl mutant co-precipitate with mitochondrial aconitase
Mitochondrial proteins obtained from control (Con) and mt-hOggl (wild-type [WT] and long α/β 317–323 [Laβ]) overexpressing A549 cells were subjected to Western blot analysis before and after immunoprecipitation (IP) using antimyc performed as described in the Experimental Procedures. As expected, mitochondrial proteins probed before IP demonstrated hOgg 1 and c-myc only in the hOgg 1 overexpressing cells (either wild-type or Lαβ mutant hOggl) despite similar levels of mitochondrial aconitase and COX IV as control cells. After IP with c-myc, mitochondrial aconitase and hOggl, but not COX IV, co-precipitated with the myc constructs containing either wild-type or Lαβ mutant hOggl.
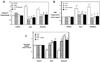
Figure 7. Mitochondria-targeted aconitase overexpression prevents oxidant-induced A549 cell DNA fragmentation and caspase-9 activation
(A) Mitochondria-targeted aconitase augments aconitase protein levels as assessed by Western blotting and (B) mitochondrial aconitase activity (n=3). (C) Mitochondria-targeted aconitase overexpression blocks oxidative stress-induced DNA fragmentation and caspase-9 activation caused by H2O2 (100 µM) or asbestos (25 µg/cm2) for 24h. * p < .05 v. no H2O2 or asbestos; † p < .05 v. empty vector cells exposed to the same dose of H2O2 or asbestos.
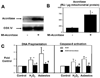
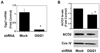
Figure 8. shRNA-mediated down-regulation of hOGGl mRNA reduces mitochondrial aconitase and augments oxidant-induced apoptosis
Cells were transfected with mock, control shRNA or hOGGl shRNA for 24 h as delineated in the Experimental Procedures. As compared to mock shRNA controls, shRNA against hOGGl reduces OGG1 mRNA levels by ~90% (n=6) (A) and decreases mitochondrial aconitase levels by ~30% (n=3) (B). As compared to Mock shRNA-treated controls (Con), DNA fragmentation caused by asbestos (5–25 µg/cm2) or H2O2 (100 µM) for 24h was augmented by shRNA against hOGGl (n=6); data expressed as fold control (mock shRNA-treated) (C) * p < .05 v. Control; † p < .05 v. Mock shRNA.
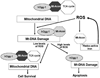
Figure 9. Hypothetical model in which mt-hOggl and aconitase interact in the setting of oxidative stress to promote cell life or death
Similar articles
- Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells.
Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, Weitzman S, Bohr VA, Kamp DW. Kim SJ, et al. J Biol Chem. 2014 Feb 28;289(9):6165-76. doi: 10.1074/jbc.M113.515130. Epub 2014 Jan 15. J Biol Chem. 2014. PMID: 24429287 Free PMC article. - β-8-Oxoguanine DNA Glycosylase Overexpression Reduces Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis Through the JNK Signaling Pathway in Human Bronchial Epithelial Cells.
Lin Z, Xu W, Li C, Wang Y, Yang L, Zou B, Gao S, Yao W, Song Z, Liu G. Lin Z, et al. DNA Cell Biol. 2017 Dec;36(12):1071-1080. doi: 10.1089/dna.2017.3769. DNA Cell Biol. 2017. PMID: 29227732 - Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2'-deoxyguanosine.
Smart DJ, Chipman JK, Hodges NJ. Smart DJ, et al. DNA Repair (Amst). 2006 Nov 8;5(11):1337-45. doi: 10.1016/j.dnarep.2006.06.001. Epub 2006 Jul 24. DNA Repair (Amst). 2006. PMID: 16861056 - The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.
Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. Kim SJ, et al. Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review. - Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis.
Liu G, Beri R, Mueller A, Kamp DW. Liu G, et al. Chem Biol Interact. 2010 Nov 5;188(2):309-18. doi: 10.1016/j.cbi.2010.03.047. Epub 2010 Apr 7. Chem Biol Interact. 2010. PMID: 20380827 Review.
Cited by
- Redox Imbalance in Idiopathic Pulmonary Fibrosis: A Role for Oxidant Cross-Talk Between NADPH Oxidase Enzymes and Mitochondria.
Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Veith C, et al. Antioxid Redox Signal. 2019 Nov 10;31(14):1092-1115. doi: 10.1089/ars.2019.7742. Epub 2019 Apr 5. Antioxid Redox Signal. 2019. PMID: 30793932 Free PMC article. Review. - The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells.
Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. Ruchko MV, et al. Free Radic Biol Med. 2011 May 1;50(9):1107-13. doi: 10.1016/j.freeradbiomed.2010.10.692. Epub 2010 Oct 20. Free Radic Biol Med. 2011. PMID: 20969951 Free PMC article. - The Sphingosine Kinase 1 Inhibitor, PF543, Mitigates Pulmonary Fibrosis by Reducing Lung Epithelial Cell mtDNA Damage and Recruitment of Fibrogenic Monocytes.
Cheresh P, Kim SJ, Huang LS, Watanabe S, Joshi N, Williams KJN, Chi M, Lu Z, Harijith A, Yeldandi A, Lam AP, Gottardi C, Misharin AV, Budinger GRS, Natarajan V, Kamp DW. Cheresh P, et al. Int J Mol Sci. 2020 Aug 5;21(16):5595. doi: 10.3390/ijms21165595. Int J Mol Sci. 2020. PMID: 32764262 Free PMC article. - Absence of the DNA repair enzyme human 8-oxoguanine glycosylase is associated with an aggressive breast cancer phenotype.
Karihtala P, Kauppila S, Puistola U, Jukkola-Vuorinen A. Karihtala P, et al. Br J Cancer. 2012 Jan 17;106(2):344-7. doi: 10.1038/bjc.2011.518. Epub 2011 Nov 22. Br J Cancer. 2012. PMID: 22108520 Free PMC article. - Enhanced mitochondrial DNA repair of the common disease-associated variant, Ser326Cys, of hOGG1 through small molecule intervention.
Baptiste BA, Katchur SR, Fivenson EM, Croteau DL, Rumsey WL, Bohr VA. Baptiste BA, et al. Free Radic Biol Med. 2018 Aug 20;124:149-162. doi: 10.1016/j.freeradbiomed.2018.05.094. Epub 2018 Jun 5. Free Radic Biol Med. 2018. PMID: 29879444 Free PMC article.
References
- Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. - PubMed
- Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5:145–152. - PubMed
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL35440/HL/NHLBI NIH HHS/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- K08 HL067835/HL/NHLBI NIH HHS/United States
- R01 HL035440/HL/NHLBI NIH HHS/United States
- HL67835-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials