Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone - PubMed (original) (raw)
Review
Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone
Yuttana Chawengsub et al. Am J Physiol Heart Circ Physiol. 2009 Aug.
Abstract
Stimulation of vascular endothelial cells with agonists such as acetylcholine (ACh) or bradykinin or with shear stress activates phospholipases and releases arachidonic acid (AA). AA is metabolized by cyclooxygenases, cytochrome P-450s, and lipoxygenases (LOs) to vasoactive products. In some arteries, a substantial component of the vasodilator response is dependent on LO metabolites of AA. Nitric oxide (NO)- and prostaglandin (PG)-independent vasodilatory responses to ACh and AA are reduced by inhibitors of LO and by antisense oligonucleotides specifically against 15-LO-1. Vasoactive 15-LO metabolites derived from the vascular endothelium include 15-hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-HEETA) that is hydrolyzed by soluble epoxide hydrolase to 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA). HEETA and THETA are endothelium-derived hyperpolarizing factors that induce vascular relaxations by activation of smooth muscle apamin-sensitive, calcium-activated, small-conductance K(+) channels causing hyperpolarization. In other arteries, the 12-LO metabolite 12-hydroxyeicosatetraenoic acid is synthesized by the vascular endothelium and relaxes smooth muscle by large-conductance, calcium-activated K(+) channel activation. Thus formation of vasodilator eicosanoids derived from LO pathways contributes to the regulation of vascular tone, local blood flow, and blood pressure.
Figures
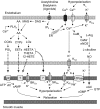
Fig. 1.
Schematic of the role of Ca2+ on relaxing factor production in vascular endothelial cells. Activation of muscarinic receptors leads to the production of inositol 1,4,5-trisphosphate (IP3), which releases Ca2+ from the endoplasmic reticulum (ER). Extracellular Ca2+ can enter the endothelial cell through nonselective Ca2+-permeable cation channels or store-operated Ca2+ channels, which are regulated by the ER Ca2+ store. An increase in Ca2+ concentration promotes K+ efflux through activated Ca2+-dependent K+ (KCa) channels, causing membrane hyperpolarization, which in turn increases Ca2+ influx. Intracellular Ca2+ binds to calmodulin (CaM) and activates nitric oxide synthase (NOS) and the production of nitric oxide (NO) or stimulates phospholipase A2 (PLA2), which results in the release of arachidonic acid (AA) from the cell plasma membrane. Free AA also can be obtained from the phospholipase C (PLC) pathway, in which diacylglycerol (DAG) is hydrolyzed at the sn-1 position by DAG lipase to monoacylglycerol (MAG), specifically 2-arachidonylglycerol (2-AG). MAG lipase or fatty acid amidohydrolase (FAAH) releases free AA from 2-AG. Free AA can be metabolized through cyclooxygenase (COX), cytochrome _P_-450 (CYP), and/or lipoxygenase (LO) pathways to produce relaxing factors. PGI2, prostacyclin; EET, epoxyeicosatrienoic acid; HEETA, hydroxyepoxyeicosatrienoic acid; THETA, trihydroxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; BKCa, large-conductance KCa channel; SKCa, apamin-sensitive KCa channel; Kir, inward rectifying K+ channel; AC, adenylyl cyclase; sGC, soluble guanylate cyclase.
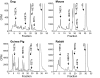
Fig. 2.
Metabolism of [14C]AA by rabbit aorta, mouse aorta, dog mesenteric arteries, and guinea pig carotid arteries. Arterial rings with an intact endothelium were incubated with indomethacin and [14C]AA. Metabolites were extracted and resolved by reverse-phase HPLC (21). Migration times of known standards are indicated by arrows above the chromatogram. CPM, counts per minute.
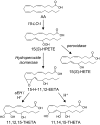
Fig. 3.
Proposed biosynthesis pathway of HEETAs and THETAs. AA is converted to 15(S)-hydroperoxyeicosatetraenoic acid [15(S)-HPETE] by 15-LO-1. 15(S)-HPETE is converted by hydroperoxide isomerases to the HEETAs. 15-Hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA) can be hydrolyzed by either soluble epoxide hydrolase (sEH) or acid to THETAs. It is hydrolyzed to 11,12,15-THETA by sEH. However, with acid hydrolysis, 15-H-11,12-EETA is hydrolyzed to both 11,12,15- and 11,14,15-THETA. The exact stereochemistry of 15-H-11,12-EETAs is not known.
Fig. 4.
Endothelium-derived hyperpolarizing factor (EDHF)-mediated responses to acetylcholine (ACh) involve 2 mechanisms acting in parallel. ACh activates intermediate-conductance KCa (IKCa) channels on endothelial cells (EC), releasing K+ that activates Kir channels on smooth muscle. ACh also releases AA in endothelial cells that is metabolized by 15-LO to 15-H-11,12-EETA and 11,12,15-THETA. THETA activates SKCa channels on smooth muscle. These 2 pathways cause smooth muscle cell membrane hyperpolarization and relaxation. Inhibitors of these enzymes and channels are indicated. PL, phospholipase; CDC, cinnamyl-3,4-dihydroxy-α-cyanocinnamate; ETYA, 5,8,11,14-eicosatetraynoic acid; NDGA, nordihydroguaiaretic acid; ChTX, charybdotoxin; SMC, smooth muscle cells.
Similar articles
- Chronic hypoxia enhances 15-lipoxygenase-mediated vasorelaxation in rabbit arteries.
Aggarwal NT, Pfister SL, Gauthier KM, Chawengsub Y, Baker JE, Campbell WB. Aggarwal NT, et al. Am J Physiol Heart Circ Physiol. 2009 Mar;296(3):H678-88. doi: 10.1152/ajpheart.00777.2008. Epub 2008 Dec 26. Am J Physiol Heart Circ Physiol. 2009. PMID: 19112096 Free PMC article. Retracted. - 11,12,15-Trihydroxyeicosatrienoic acid mediates ACh-induced relaxations in rabbit aorta.
Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. Campbell WB, et al. Am J Physiol Heart Circ Physiol. 2003 Dec;285(6):H2648-56. doi: 10.1152/ajpheart.00412.2003. Epub 2003 Aug 7. Am J Physiol Heart Circ Physiol. 2003. PMID: 12907422 - Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta.
Chawengsub Y, Aggarwal NT, Nithipatikom K, Gauthier KM, Anjaiah S, Hammock BD, Falck JR, Campbell WB. Chawengsub Y, et al. Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1348-56. doi: 10.1152/ajpheart.01326.2007. Epub 2008 Jan 11. Am J Physiol Heart Circ Physiol. 2008. PMID: 18192225 - Inducible endothelium-derived hyperpolarizing factor: role of the 15-lipoxygenase-EDHF pathway.
Campbell WB, Gauthier KM. Campbell WB, et al. J Cardiovasc Pharmacol. 2013 Mar;61(3):176-87. doi: 10.1097/FJC.0b013e31828165db. J Cardiovasc Pharmacol. 2013. PMID: 23249676 Free PMC article. Review. - Epoxyeicosatrienoic acids and endothelium-dependent responses.
Campbell WB, Fleming I. Campbell WB, et al. Pflugers Arch. 2010 May;459(6):881-95. doi: 10.1007/s00424-010-0804-6. Epub 2010 Mar 12. Pflugers Arch. 2010. PMID: 20224870 Free PMC article. Review.
Cited by
- CYP2C19 Loss-of-Function is an Associated Risk Factor for Premature Coronary Artery Disease: A Case-Control Study.
Chen W, Liu Y, Deng X, Li B, Wang H, Wei G, Chen K, Wang S. Chen W, et al. Int J Gen Med. 2024 Nov 3;17:5049-5058. doi: 10.2147/IJGM.S486187. eCollection 2024. Int J Gen Med. 2024. PMID: 39512259 Free PMC article. - CYP2C19 Poor Metabolizer Status and High System Inflammation Response Index are Independent Risk Factors for Premature Myocardial Infarction: A Hospital-Based Retrospective Study.
Han W, Xiong N, Zhong R, Pan Z. Han W, et al. Int J Gen Med. 2024 Oct 28;17:4959-4969. doi: 10.2147/IJGM.S489235. eCollection 2024. Int J Gen Med. 2024. PMID: 39494358 Free PMC article. - CYP2C19 loss-of-function variants are independent risk factors for premature cerebral infarction: a hospital based retrospective study.
Shi Y, Yang Y, Feng M, Wu H. Shi Y, et al. BMC Cardiovasc Disord. 2024 Oct 29;24(1):602. doi: 10.1186/s12872-024-04269-0. BMC Cardiovasc Disord. 2024. PMID: 39472784 Free PMC article. - Mechanisms of Abnormal Lipid Metabolism in the Pathogenesis of Disease.
Xu L, Yang Q, Zhou J. Xu L, et al. Int J Mol Sci. 2024 Aug 2;25(15):8465. doi: 10.3390/ijms25158465. Int J Mol Sci. 2024. PMID: 39126035 Free PMC article. Review. - Bazi Bushen ameliorates age-related energy metabolism dysregulation by targeting the IL-17/TNF inflammatory pathway associated with SASP.
Shen X, Li M, Li Y, Jiang Y, Niu K, Zhang S, Lu X, Zhang R, Zhao Z, Zhou L, Guo Z, Wang S, Wei C, Chang L, Hou Y, Wu Y. Shen X, et al. Chin Med. 2024 Apr 9;19(1):61. doi: 10.1186/s13020-024-00927-9. Chin Med. 2024. PMID: 38594761 Free PMC article.
References
- Aggarwal N, Pfister SL, Campbell WB. Hypercholesterolemia enhances 15-lipoxygenase mediated vasorelaxation and acetylcholine-induced hypotension. Arterioscler Thromb Vasc Biol 28: 2209–2215, 2008. - PubMed
- Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension 51: 246–251, 2008. - PubMed
- Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. Am J Physiol Heart Circ Physiol 292: H1033–H1041, 2007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources