Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor - PubMed (original) (raw)
Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor
Jeffrey A DeGrasse et al. Mol Cell Proteomics. 2009 Sep.
Abstract
The nuclear pore complex (NPC) is a macromolecular assembly embedded within the nuclear envelope that mediates bidirectional exchange of material between the nucleus and cytoplasm. Our recent work on the yeast NPC has revealed a simple modularity in its architecture and suggested a common evolutionary origin of the NPC and vesicle coating complexes in a progenitor protocoatomer. However, detailed compositional and structural information is currently only available for vertebrate and yeast NPCs, which are evolutionarily closely related. Hence our understanding of NPC composition in a full evolutionary context is sparse. Moreover despite the ubiquitous nature of the NPC, sequence searches in distant taxa have identified surprisingly few NPC components, suggesting that much of the NPC may not be conserved. Thus, to gain a broad perspective on the origins and evolution of the NPC, we performed proteomics analyses of NPC-containing fractions from a divergent eukaryote (Trypanosoma brucei) and obtained a comprehensive inventory of its nucleoporins. Strikingly trypanosome nucleoporins clearly share with metazoa and yeast their fold type, domain organization, composition, and modularity. Overall these data provide conclusive evidence that the majority of NPC architecture is indeed conserved throughout the Eukaryota and was already established in the last common eukaryotic ancestor. These findings strongly support the hypothesis that NPCs share a common ancestry with vesicle coating complexes and that both were established very early in eukaryotic evolution.
Figures
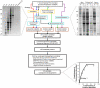
Fig. 1.
Summary flow chart of biochemical, mass spectrometric, and bioinformatics methods used to identify putative T. brucei nucleoporins and transport factors. Strategies 1–5 are indicated by the red, blue, green, purple, and black colored arrows, respectively. The boxes are colored as follows: gold, protein recovery steps; light blue, protein separation steps; and brown, mass spectrometry techniques. Following mass spectrometry, the bioinformatics strategy outlined here identified 30 putative TbNPC-associated proteins from the initial pool of 757 identified proteins in the TbNEP. SDS-PAGE of fractions from a representative hydroxyapatite separation of the nuclear envelope fraction is shown at the top left. FW, flow-through and wash. Concentrations of phosphate in the elution buffer are indicated above the gel lanes, and apparent molecular masses (in kDa) are shown to the left of the gel. SDS-PAGE of T. brucei NE proteins that were subjected to chemical extraction is shown at the top right. The three extractions (base, salt and detergent (Det.), and heparin) are separated by vertical dashed lines. The pellet (P) and supernatant (S) are indicated. The number of Nups versus the total number of proteins identified with each successive strategy is depicted in the scatter plot (bottom right). Although the total number of proteins identified increases dramatically with further experimentation, the number of NPC-associated proteins levels off after four strategies. 1-D, one-dimensional.
Fig. 2.
Validation of candidate T. brucei Nups. A, one copy of open reading frame Tb11.03.0140 (TbNup158) was genomically tagged at the COOH terminus with GFP. A montage of 21 confocal planes from the analysis of a TbNup158-tagged trypanosome in late anaphase is shown; each z-slice is 150 nm thick. There are ∼150 puncta associated with the nuclear envelope in this example. B, fluorescent microscopy gallery of COOH-terminal genomically labeled TbNups and corresponding 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) fluorescence to visualize the DNA. Apart from TbSec13, which was labeled using the 3×HA epitope and visualized with a mouse monoclonal anti-hemagglutinin antibody at 1:1000, all other open reading frames were tagged with GFP. Scale bars, 2 μm.
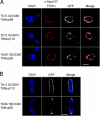
Fig. 3.
TbNup92 exhibits cell cycle-dependent localization. A, a rabbit polyclonal antibody against HsNup107 (48) was used to stain a trypanosome cell bearing tagged TbNup89. Co-localization of these signals further supports assignment of the puncta as the trypanosome NPC (top). Two coiled coil TbNups, TbNup110 and TbNup92, only partially co-localize with this antibody and are found immediately to the nuclear side of the NPCs and adjacent to them, suggesting association with the nuclear basket of the NPC and consistent with potential similarity to Tpr (bottom). B, TbNup110-GFP and TbNup92-GFP, visualized in mitotic cells, demonstrate that although TbNup110 remains associated with the NPC throughout mitosis TbNup92 relocates to opposite poles in a region similar to the spindle attachment site. Scale bar, 2 μm. DAPI, 4′,6-diamino-2-phenylindole dihydrochloride.
Fig. 4.
Predicted secondary structure features, fold, and location for validated TbNups. The ruler at the top indicates residue number. Within a map, the horizontal black line represents the polypeptide length of the Nup with the NH2 terminus to the left. The y axis indicates the confidence score of the predicted secondary structure element. Predicted α-helices are shaded in magenta, predicted β-sheets are in blue, and predicted coiled coil regions are in red. The vertical orange lines below the primary structure indicate FG dipeptides. Representative models of the Nup domains, colored according to their fold type, are shown to the left. The TbNups are binned according to their predicted fold type, and thus probable function, within the TbNPC; possible yeast and human homologs are indicated in the right-most column. Predicted positions of each Nup or Nup structural class within the NPC are shown at right based on the architecture as determined for S. cerevisiae.
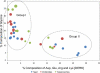
Fig. 5.
Correlation between the frequency of glycine and charged residues in trypanosome, yeast, and human FG repeat Nups. The percent composition of Gly is plotted against Asp, Glu, Arg, and Lys (DERK) residue frequency. Each data point represents an FG Nup from either S. cerevisiae (blue) or H. sapiens (red) or a candidate FG Nup from T. brucei (green). The diameter of each data point is directly proportional to the phenylalanine concentration within the respective Nup. FG Nups tend to cluster into two groups: high Gly, low DERK (Group I) and low Gly, high DERK (Group II). The average natural occurrence (in vertebrates) for Phe is ∼4% and for Gly is ∼7%, and the sum natural occurrence for the charged residues is ∼23%.
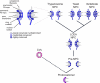
Fig. 6.
A model for the evolutionary origin of the NPC. A primitive coating complex (bottom, purple) evolved into numerous vesicle coating complexes (pink) and a simpler pre-NPC, which through duplication and divergence of its constituents produced a complex and elaborate NPC in the LCEA. The composition and architecture of the contemporary NPC throughout the Eukaryota is largely conserved with species-specific adaptations arising primarily by divergent evolution. The inferred degrees of conservation of the indicated different architectural elements of the trypanosome, yeast, and vertebrate NPC (with vertebrate set as the standard) is shown in shades of blue based on the analysis presented here. CVs, coated vesicles.
Similar articles
- Simple fold composition and modular architecture of the nuclear pore complex.
Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A. Devos D, et al. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2172-7. doi: 10.1073/pnas.0506345103. Epub 2006 Feb 6. Proc Natl Acad Sci U S A. 2006. PMID: 16461911 Free PMC article. - Interactome Mapping Reveals the Evolutionary History of the Nuclear Pore Complex.
Obado SO, Brillantes M, Uryu K, Zhang W, Ketaren NE, Chait BT, Field MC, Rout MP. Obado SO, et al. PLoS Biol. 2016 Feb 18;14(2):e1002365. doi: 10.1371/journal.pbio.1002365. eCollection 2016 Feb. PLoS Biol. 2016. PMID: 26891179 Free PMC article. - [Nuclear pores: from yeast to higher eukaryotes].
Doye V. Doye V. J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French. - Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex.
Mans BJ, Anantharaman V, Aravind L, Koonin EV. Mans BJ, et al. Cell Cycle. 2004 Dec;3(12):1612-37. doi: 10.4161/cc.3.12.1316. Epub 2004 Dec 20. Cell Cycle. 2004. PMID: 15611647 - The nuclear pore complex core scaffold and permeability barrier: variations of a common theme.
Hayama R, Rout MP, Fernandez-Martinez J. Hayama R, et al. Curr Opin Cell Biol. 2017 Jun;46:110-118. doi: 10.1016/j.ceb.2017.05.003. Epub 2017 Jun 15. Curr Opin Cell Biol. 2017. PMID: 28624666 Free PMC article. Review.
Cited by
- Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly.
Vollmer B, Schooley A, Sachdev R, Eisenhardt N, Schneider AM, Sieverding C, Madlung J, Gerken U, Macek B, Antonin W. Vollmer B, et al. EMBO J. 2012 Oct 17;31(20):4072-84. doi: 10.1038/emboj.2012.256. Epub 2012 Sep 7. EMBO J. 2012. PMID: 22960634 Free PMC article. - Telomeres, tethers and trypanosomes.
Field MC, Horn D, Alsford S, Koreny L, Rout MP. Field MC, et al. Nucleus. 2012 Nov-Dec;3(6):478-86. doi: 10.4161/nucl.22167. Epub 2012 Sep 19. Nucleus. 2012. PMID: 22992703 Free PMC article. - A jumbo problem: mapping the structure and functions of the nuclear pore complex.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2012 Feb;24(1):92-9. doi: 10.1016/j.ceb.2011.12.013. Epub 2012 Feb 8. Curr Opin Cell Biol. 2012. PMID: 22321828 Free PMC article. Review. - Nuclear Pore Complex Components in the Malaria Parasite Plasmodium berghei.
Kehrer J, Kuss C, Andres-Pons A, Reustle A, Dahan N, Devos D, Kudryashev M, Beck M, Mair GR, Frischknecht F. Kehrer J, et al. Sci Rep. 2018 Jul 26;8(1):11249. doi: 10.1038/s41598-018-29590-5. Sci Rep. 2018. PMID: 30050042 Free PMC article. - The nuclear pore complex and nuclear transport.
Wente SR, Rout MP. Wente SR, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a000562. doi: 10.1101/cshperspect.a000562. Epub 2010 Jul 14. Cold Spring Harb Perspect Biol. 2010. PMID: 20630994 Free PMC article. Review.
References
- Dacks J. B., Field M. C. (2007) Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci 120, 2977–2985 - PubMed
- Cavalier-Smith T. (1975) The origin of nuclei and of eukaryotic cells. Nature 256, 463–468 - PubMed
- Suntharalingam M., Wente S. R. (2003) Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell 4, 775–789 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR022220/RR/NCRR NIH HHS/United States
- P41 RR000862/RR/NCRR NIH HHS/United States
- U54 RR022220/RR/NCRR NIH HHS/United States
- 082813/Z/07/Z/WT_/Wellcome Trust/United Kingdom
- R01 GM062427/GM/NIGMS NIH HHS/United States
- GM062427/GM/NIGMS NIH HHS/United States
- RR00862/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases