Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc - PubMed (original) (raw)
Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc
Rachel K Smith-Bolton et al. Dev Cell. 2009 Jun.
Abstract
The study of regeneration would be aided greatly by systems that support large-scale genetic screens. Here we describe a nonsurgical method for inducing tissue damage and regeneration in Drosophila larvae by inducing apoptosis in the wing imaginal disc in a spatially and temporally regulated manner. Tissue damage results in localized regenerative proliferation characterized by altered expression of patterning genes and growth regulators as well as a temporary loss of markers of cell fate commitment. Wingless and Myc are induced by tissue damage and are important for regenerative growth. Furthermore, ectopic Myc enhances regeneration when other growth drivers tested do not. As the animal matures, the ability to regenerate is lost and cannot be restored by activation of Wingless or Myc. This system is conducive to forward genetic screens, enabling an unbiased search for genes that regulate both the extent of and the capacity for regeneration.
Figures
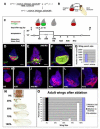
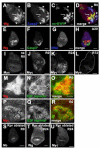
Figure 1. A novel genetic system induces ablation and regeneration
(A) The genetic cross whose progeny undergo ablation in the wing imaginal disc. In specific experiments w1118;+;+ is replaced with other genotypes, such as w1118;+;UASEGFP in (E) and (F). (B) Diagram showing regions of the wing-imaginal disc and the corresponding adult structures. (C) The protocol used to study ablation and regeneration. Animals were raised at 18° and shifted to 30° for 40 hours during early third instar larval development. Unless otherwise specified, this shift began at 7d AEL. Larvae were returned to 18° and allowed to pupariate and eclose or were dissected at the time points noted during the Ablation (A) or Recovery (R) periods. Drawings not to scale. (D) An antibody for cleaved Caspase 3 (green) marks dying tissue and debris at A20, which is no longer recognized by the wing pouch marker anti-Nubbin (red). (E-G) Comparison of pouch size in ablated (E) and mock-ablated (F) wing discs. EGFP (green) marks the _rnGAL4_-expressing tissue that had not undergone apoptosis and remained in the epithelium (arrow) as well as debris from _rnGAL4_-expressing tissue that has undergone apoptosis and is found between the folds of the disc (arrowhead). Nb (red) marks the wing pouch. na = non ablating. (G) Quantification of extent of ablation as measured by number of Nb positive cells. Discs were from two separate experiments. Control n=2, Ablating n=10. Error bars mark one standard deviation. (H-M) Size of the wing pouch, stained with anti-Nb, at different time points in the Ablation and Recovery periods. (N) The range of adult wing sizes observed in adult animals that had undergone the ablation protocol compared to the size of a normal wing. (O) Adult wing sizes observed under different experimental conditions. Ablation and recovery resulted in a range of wing sizes, n = 6 experiments, 356 wings. Error bars mark SEM. All scale bars are 100μm.
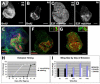
Figure 2. Regenerative growth is localized at the site of ablation
(A-B) BrdU incorporation marked cells in S phase in non-ablating (A) and ablated (B) wing discs at R0. (C-D) E2F reporter (PCNA-GFP) marked cells that had cycled through S-phase in non-ablating (C) and ablated (D) discs. (E) The E2F reporter (green) marked proliferating cells largely within the Nubbin-expressing (red) wing pouch at R0. (F) _lacZ_-marked clones induced at R0 and visualized at R72. Insets show a clone from the notum (left) and a clone from the pouch (right). (G) _lacZ_-marked clones in a non-ablating disc induced 72 hours before dissection at late wandering stage. Insets show a clone from the notum (left) and a clone from the pouch (right). (H) Time to eclosion for animals with non-ablating and ablated wing discs in one representative experiment (n = 62 wings). (I) Comparison of wing sizes on animals with ablated discs that eclosed on successive days in the same experiment as H. All scale bars are 100μm.
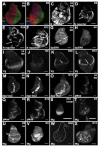
Figure 3. Wing disc patterning is altered during ablation and regeneration
(A-B) Ci (red) and En (green) marked the anterior and posterior compartments, respectively in non-ablating (A) and ablated discs (B) at R0. (C-F) E(Spl) Mβ-CD2 as a reporter for Notch signaling. (C) Non-ablating disc at R0. A stripe of N signaling was seen at the DV boundary (arrow) and N signaling was seen in the presumptive intervein regions (arrowhead). (D) Ablated disc at R0. (E) Ablated disc at R24. (F) Ablated disc at R72. All images recorded with the same gain. (G-H) di-phosphorylated ERK as a measure of RTK signaling. (G) Non-ablating disc at R0. dpERK was seen in the presumptive wing veins (arrows). (H) Ablated disc at R0. (I-L) Vestigial expression. (I) Non-ablating disc at R0. (J) Ablated disc at R0. (K) Ablated disc at R48. (L) Ablated disc at R72. Arrows mark discontinuity in expression. (M-O) dpp-lacZ expression. (M) Non-ablating disc at R0. (N) Ablated disc at R0. (O) Ablated disc at R72. (P-R) Phosphorylated Mad as a measure of Dpp signaling. (P) Non-ablating disc at R0. (Q) Ablated disc at R0. (R) Ablated disc at R72. (S-X) Wingless expression. (S) Wingless in a second instar disc was expressed throughout the pouch. (T) Wingless in an early third instar disc (A0) was expressed in a stripe at the DV boundary (arrowhead) and a ring around the pouch (arrow). (U) Non-ablating disc at T0. (V) Ablated disc at T0. (W) Ablated disc at R48. (X) Ablated disc at T72. All scale bars are 100μm.
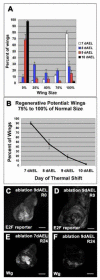
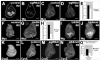
Figure 4. Regeneration only occurred during a specific developmental window
(A) Adult wing sizes observed after ablations beginning on 7, 8, 9, or 10 dAEL. 7dAEL n = 6 experiments, 356 wings. 8dAEL n = 5 experiments 178 wings. 9dAEL n = 3 experiments 119 wings. 10dAEL n = 4 experiments 158 wings. (B) The number of wings 75 to 100% of normal wing size as a measure of regenerative potential. Numbers of experiments and wings are the same as in panel (A). (C-D) E2F reporter (PCNA-GFP) in discs that had undergone ablation at 9dAEL. (E-F) Wg expression at R24 in a disc that had undergone ablation at 7dEAL (E), and a disc that had undergone ablation at 9dAEL (F). Error bars=SEM.
Figure 5. Wg and Myc are unpregulated in regenerating wing discs
(A-D) Wg (using Anti-Wg antibody) expression at A40/R0. At time R0, low Wg expression was associated with cellular debris (arrowheads), high Wg expression was seen among cells that remained in the disc epithelium (arrow). (A) Wg (B) cleaved Caspase 3 (C) rn>EYFP (D) merge. (E-G) Wg expression at A20. Apoptotic tissue is not associated with Wg. (E) Wg (F) cleaved Caspase 3 (G) DNA (H) merge. (I-K) Myc expression (using anti-Myc antibody) in a non-ablating disc at R0 (I) an ablated disc at R0 (J) an ablated disc at R24 (K) and an ablated disc at R72 (L). (M-O) High Wg expression was associated with cells showing high levels of the E2F reporter PCNA-GFP. (M) Wg (N) E2F reporter (O) merge (P-Q) Elevated levels of Myc coincided with elevated levels of the E2F reporter in an ablated disc at R0. (P) Myc (Q) E2F reporter (R) merge. (S-U) Wing discs ablated with rnGAL4, UASreaper. (S) Nubbin, (T) Wg and (U) Myc. All scale bars are 100μm.
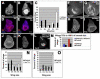
Figure 6. Wg promotes regenerative growth through Myc and Cyclin E
(A-B) Wg protein levels at R0 in a control ablated disc (A) and an ablated disc expressing UASwgRNAi (B). Arrows point to comparable Wg levels in the notum. (C-E) Myc levels were reduced in ablated discs at R0 that expressed UASwgRNAi. (C) control (D) UASwgRNAi (E) Quantification of anti-Myc staining in pouch region, n=3 discs for each. (F-H) Myc levels were reduced in the pouch of an ablated disc at R0 expressing UASNFL. (F) control (G) UASNFL. H) Quantification of anti-Myc staining, n = 3 discs for each. (I-J) Wg expression in ablated discs expressing UASNFL (I) or UASmnt (J). (K-L) Cyclin E protein in a non ablating disc (K), ablated disc (L), ablated disc expressing UASwgRNAi (M), and ablated disc expressing UASmnt (N). (O) Expression of UASNFL in discs in which ablation was induced at 8dAEL led to reduced adult wing sizes. n = 4 experiments, 132 wings (UASNFL) and 194 wings (control). All scale bars are 100μm. Error bars = SEM.
Figure 7. Myc potentiates regenerative growth
(A-B) Myc protein levels at R0 in an ablated disc (A) and an ablated disc expressing UASmyc (B). (C) Expression of UASmyc in ablated discs resulted in an increase in adult wing sizes. n = 3 experiments, 240 wings (UASmyc) and 78 wings (control). (D-E) Nb marked the wing pouch at R0 in an ablated disc (D), and an ablated disc expressing UASmyc (E). (F-G) Cleaved caspase 3 at R0 as a marker for tissue undergoing apoptosis in an ablated disc (F) and an ablated disc expressing UASmyc (G). (H-I) Phospho-Histone H3 at R0 as a marker for mitotic cells in an ablated disc (H) and an ablated disc expressing UASmyc (I). Lines denote location of remaining Nubbin staining tissue. (J-L) Cyclin D expression at R0 in a non-ablating disc (J) an ablated disc (K) and an ablated disc expressing UAScycD, UAScdk4 (L). (M) Percent of adult wings 75% to 100% of normal size was increased slightly when UAScycD, UAScdk4 was expressed (n = 5 experiments, UAScycD, UAScdk4 = 175 wings, control = 190 wings), and increased considerably when UASmyc was expressed (n same as panel C). (N-O) Heterozygosity for cic474 resulted in an increase in adult wing sizes when ablation was induced by UASeiger (N) (n = 3 experiments, cic474 = 205 wings, control = 252 wings) or UASreaper (O) (n = 4 experiments, cic474 = 178 wings, control = 378 wings). All scale bars are 100μm. Error bars = SEM.
Comment in
- Genetic DISC-section of regeneration in Drosophila.
Nachtrab G, Poss KD. Nachtrab G, et al. Dev Cell. 2009 Jun;16(6):777-8. doi: 10.1016/j.devcel.2009.05.015. Dev Cell. 2009. PMID: 19531347 Free PMC article.
Similar articles
- Genetic DISC-section of regeneration in Drosophila.
Nachtrab G, Poss KD. Nachtrab G, et al. Dev Cell. 2009 Jun;16(6):777-8. doi: 10.1016/j.devcel.2009.05.015. Dev Cell. 2009. PMID: 19531347 Free PMC article. - Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium.
Huang H, Kornberg TB. Huang H, et al. Elife. 2015 May 7;4:e06114. doi: 10.7554/eLife.06114. Elife. 2015. PMID: 25951303 Free PMC article. - Ecdysone exerts biphasic control of regenerative signaling, coordinating the completion of regeneration with developmental progression.
Karanja F, Sahu S, Weintraub S, Bhandari R, Jaszczak R, Sitt J, Halme A. Karanja F, et al. Proc Natl Acad Sci U S A. 2022 Feb 1;119(5):e2115017119. doi: 10.1073/pnas.2115017119. Proc Natl Acad Sci U S A. 2022. PMID: 35086929 Free PMC article. - Regeneration and transdetermination in Drosophila imaginal discs.
Worley MI, Setiawan L, Hariharan IK. Worley MI, et al. Annu Rev Genet. 2012;46:289-310. doi: 10.1146/annurev-genet-110711-155637. Epub 2012 Aug 29. Annu Rev Genet. 2012. PMID: 22934642 Review. - Imaginal disc regeneration takes flight.
Hariharan IK, Serras F. Hariharan IK, et al. Curr Opin Cell Biol. 2017 Oct;48:10-16. doi: 10.1016/j.ceb.2017.03.005. Epub 2017 Apr 1. Curr Opin Cell Biol. 2017. PMID: 28376317 Free PMC article. Review.
Cited by
- The role of apoptosis-induced proliferation for regeneration and cancer.
Ryoo HD, Bergmann A. Ryoo HD, et al. Cold Spring Harb Perspect Biol. 2012 Aug 1;4(8):a008797. doi: 10.1101/cshperspect.a008797. Cold Spring Harb Perspect Biol. 2012. PMID: 22855725 Free PMC article. Review. - Drosophila activated Cdc42 kinase has an anti-apoptotic function.
Schoenherr JA, Drennan JM, Martinez JS, Chikka MR, Hall MC, Chang HC, Clemens JC. Schoenherr JA, et al. PLoS Genet. 2012;8(5):e1002725. doi: 10.1371/journal.pgen.1002725. Epub 2012 May 17. PLoS Genet. 2012. PMID: 22615583 Free PMC article. - JNK-mediated Slit-Robo signaling facilitates epithelial wound repair by extruding dying cells.
Iida C, Ohsawa S, Taniguchi K, Yamamoto M, Morata G, Igaki T. Iida C, et al. Sci Rep. 2019 Dec 20;9(1):19549. doi: 10.1038/s41598-019-56137-z. Sci Rep. 2019. PMID: 31863086 Free PMC article. - Distinct signaling signatures drive compensatory proliferation via S-phase acceleration.
Crucianelli C, Jaiswal J, Vijayakumar Maya A, Nogay L, Cosolo A, Grass I, Classen AK. Crucianelli C, et al. PLoS Genet. 2022 Dec 15;18(12):e1010516. doi: 10.1371/journal.pgen.1010516. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36520882 Free PMC article. - The early history of the eye-antennal disc of Drosophila melanogaster.
Weasner BP, Kumar JP. Weasner BP, et al. Genetics. 2022 May 5;221(1):iyac041. doi: 10.1093/genetics/iyac041. Genetics. 2022. PMID: 35460415 Free PMC article.
References
- Abbott LC, Karpen GH, Schubiger G. Compartmental restrictions and blastema formation during pattern regulation in Drosophila imaginal leg discs. Dev Biol. 1981;87:64–75. - PubMed
- Adler PN, MacQueen M. Cell proliferation and DNA replication in the imaginal wing disc of Drosophila melanogaster. Dev Biol. 1984;103:28–37. - PubMed
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. - PubMed
- Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM072252/GM/NIGMS NIH HHS/United States
- R01 GM085576-01/GM/NIGMS NIH HHS/United States
- GM085576/GM/NIGMS NIH HHS/United States
- 5F32GM072252/GM/NIGMS NIH HHS/United States
- R01 GM085576/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials