Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing - PubMed (original) (raw)
Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing
Orly Laufman et al. EMBO J. 2009.
Abstract
The crucial roles of Sec1/Munc18 (SM)-like proteins in membrane fusion have been evidenced in genetic and biochemical studies. SM proteins interact directly with SNAREs and contribute to SNARE pairing by a yet unclear mechanism. Here, we show that the SM protein, Sly1, interacts directly with the conserved oligomeric Golgi (COG) tethering complex. The Sly1-COG interaction is mediated by the Cog4 subunit, which also interacts with Syntaxin 5 through a different binding site. We provide evidence that disruption of Cog4-Sly1 interaction impairs pairing of SNAREs involved in intra-Golgi transport thereby markedly attenuating Golgi-to-ER retrograde transport. These results highlight the mechanism by which SM proteins link tethering to SNAREpin assembly.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
The interaction between the COG complex and Sly1 is mediated by the Cog4 subunit. (A) An endogenous COG complex interacts with Sly1. HeLa cell lysate was subjected to immunoprecipitation (IP) with anti-Cog3, anti-Cog7 or anti-Cog4 specific antibodies. Preimmune (P.I.) sera of Cog3 and Cog4 were used as a control. The presence of Sly1 in the immunocomplexes of the indicated COG subunits was determined by immunobloting (IB) with anti-Sly1 antibody. The interaction between the different COG subunits was assessed by immunobloting with the indicated anti-Cog antibodies. (B) Sly1 interacts with endogenous COG subunits. HeLa cells were transiently transfected with expression vector encoding Myc-tagged Sly1. Sly1-Myc was immunoprecipitated by anti-Myc antibody and the presence of Cog3, Cog7 or Cog4 in Sly1 immunocomplexes was determined by immunoblotting with the indicated anti-Cog antibodies. (C) Cog4 interacts with recombinant GST-Sly1. HEK293 cells were transiently transfected with expression vectors encoding the eight different subunits of the COG complex as Myc-tagged proteins. The cell lysates were incubated with either GST or GST-Sly1 bound to glutathione-agarose beads. The samples were washed and then resolved by SDS–PAGE, transferred to a nitrocellulose membrane and immunoblotted with anti-Myc antibody (left panel). The expression level of each COG subunit was determined by western blotting with anti-Myc antibody (right panel). (D) Cog4-Myc interacts with Sly1-HA. HEK293 cells were either transiently transfected with an expression vector encoding Sly1-HA or cotransfected with Sly1-HA and the different Cog-Myc subunits. The COG subunits were immunoprecipitated with anti-Myc antibody, and their association with Sly1-HA was determined by immunoblotting with anti-HA antibody. The expression level of the transfected proteins was assessed by immunoblotting of total cell lysates with the indicated antibodies (lower panels).
Figure 2
Sly1 interacts with an N-terminal fragment of Cog4. HEK293 cells were transiently cotransfected with expression vectors encoding the indicated Cog4-truncated mutants (A, C) as Myc-tagged proteins together with Sly1-HA. The interaction between these truncated mutants and Sly1 was determined by immunoprecipitation with anti-Myc antibody and immunoblotting with anti-HA antibody. The numbers indicate the aa residues. The expression level of the transfected proteins was assessed by immunoblotting of total cell lysates with the indicated antibodies (A, C; lower panels). (B) A direct interaction between the N-terminal fragment of Cog4 and Sly1 was determined by binding of a recombinant His-tagged Cog4 fragment (aa 1–231) to either recombinant GST or GST-Sly1 immobilized on glutathione-agarose beads, followed by immunoblotting with anti-His antibody. (D) The N-terminal fragment of Cog4 consists of distinct binding sites for Sly1 and Syntaxin 5. The indicated Cog4-truncated mutants were expressed as GST-fusion proteins in HEK293 cells with either Sly1-HA or Syntaxin 5-HA. The cell lysates were subjected to glutathione-agarose beads (GB) pull down. Interactions between the Cog4-truncated mutants and either Sly1 or Syntaxin 5 were determined by immunoblotting with anti-HA antibody. Mammalian GST (mGST) was used as a control, whereas the expression level of each truncated mutant was determined by immunoblotting with anti-GST antibody.
Figure 3
Glutamic acids at positions 53 and 71 of the human Cog4 are critical for Sly1 binding. (A) Multiple sequence alignment of Cog4 from different species. The sequences were aligned using ClustalW. Black and gray background represents degree of similarity. Asterisks mark highly conserved residues that have been mutated. Hs: Homo sapiens, Mm: Mus musculus, Gg: Gallus gallus, Dr: Danio rerio, Sp: Strongylocentrotus purpuratus, Dm: Drosophila melanogaster, Ce: Caenorhabditis elegans, At: Arabidopsis thaliana, Sc: Saccharomyces cerevisiae. (B) Interaction between Sly1-HA and Myc-tagged Cog4 fragments (aa 1–231) containing the indicated point mutations was assessed by coimmunoprecipitation using anti-Myc antibody for immunoprecipitation and anti-HA antibody for immunoblotting. (C) Substitution of glutamic acids at positions 53 and 71 by alanine abolished the interaction between Sly1 and Cog4, with no detectable effect on the Syntaxin 5–Cog4 interaction (D). The interaction between the indicated Myc-tagged Cog4 mutants and either Sly1-HA or Syntaxin 5-HA was determined by coimmunoprecipitation using anti-Myc antibody for immunoprecipitation and anti-HA antibody for immunoblotting. The expression level of the transfected proteins was assessed by immunoblotting of total cell lysates with the indicated antibodies (lower panels, B–D).
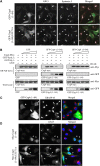
Figure 4
The N-terminal fragment of Cog4 disrupts the interaction between Cog4 and Sly1 and the colocalization of GS15 with Syntaxin 5. (A) GFP-tagged fragment consisting of the first 84 aa of either the wild-type Cog4 or the E53/71A double mutant was transiently transfected into HeLa cells. Two days later, the cells were fixed and double-immunostained with anti-GS15 and anti-Syntaxin 5 antibodies. Shown are representative confocal images of cells expressing the GFP-tagged Cog4 fragments (green) along with GS15 (red) and Syntaxin 5 (light blue). As shown, colocalization of GS15 and Syntaxin 5 was impaired in cells expressing moderate levels of the wild-type GFP-Cog4 (aa 1–84). Higher levels of expression caused Golgi fragmentation (C), as determined by immunostaining with anti-GRASP-65 antibody. Cells expressing a high level of these fragments are marked by arrows, whereas those expressing a low level are marked by arrowheads. Scale bar, 10 μm. (B) The GFP-Cog4 fragment (aa 1–84) inhibits the interaction between Cog4 and Sly1. Equal amounts of cell lysate of HEK293 cells expressing the Cog4-Myc were mixed with increasing amounts of cell lysates that were prepared from either HEK293 cells or HEK293 cells expressing the GFP-Cog4 (1–84) fragment or GFP. Equal volumes of the cell lysates mixtures were then incubated with recombinant GST, GST-Sly1 or GST-Syntaxin 5 immobilized on glutathione-agarose beads (GB pull-down), as indicated. The samples were washed and then resolved by SDS–PAGE, transferred to a nitrocellulose membrane and immunoblotted with the indicated antibodies. As shown, the GFP-Cog4 (1–84) fragment inhibited the binding of Cog4-Myc to GST-Sly1 in a concentration-dependent manner but had no effect on Cog4-Myc binding to GST-Syntaxin 5 (upper panels). The expression level of Cog4-Myc, the GFP-Cog4 (1–84) fragment or GFP in the lysates mixtures was determined by western blotting with either anti-Myc or anti-GFP antibodies (lower panels). (D) The Myc-tagged Cog4 fragment (aa 1–84) of wild-type Cog4 but not of the E53/71A double mutant disrupts the Golgi-targeting of GS15, as shown by double-immunostaining of fixed HeLa cells expressing the indicated Cog4 fragments with anti-Myc (green) and anti-GS15 (red) antibodies. Scale bar, 10 μm.
Figure 5
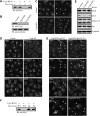
Wild-type Cog4, but not the E53/71A double mutant, restores the Golgi-targeting of SNAREs in Cog4-depleted cells. (A) HeLa cells were transiently cotransfected with shRNA construct encoding siRNA of the human Cog4 together with expression vectors encoding either Myc-tagged Cog3 or Cog4. The specificity of this siRNA was determined by immunoblotting with anti-Myc antibody. Stable HeLa cell lines depleted of Cog4 (Cog4-KD) were established and assessed for Cog4 expression by immunoblotting (B) and immunofluorescence (C) using anti-Cog4 antibody. As shown (C), depletion of Cog4 impairs the compact organization of the Golgi complex. Scale bar, 10 μm. (D) Localization of the SNARE proteins GS15, GS28 and Syntaxin 5 was determined in control and Cog4-knock-down cells by immunofluorescence analysis using the corresponding antibodies. Shown are representative confocal images. Scale bar, 10 μm. (E) Expression levels of GS15, GS28, Syntaxin 5 and Sly1 in control and Cog4-depleted HeLa cells were determined by immunoblotting using the indicated antibodies. The two bands of Syntaxin 5 represent its two isoforms. (F) Silent mutations within the RNAi targeting sequence of Cog4 protect its expression from its RNAi. HeLa cells were transiently cotransfected with the shRNA construct along with Myc-tagged of either the wild-type or the silent Cog4 mutant. Expression of Cog4 was determined by western blotting with anti-Myc antibody. (G) The wild-type Cog4, but not the E53/71A double mutant, restores the targeting of GS15 and GS28 to the Golgi in Cog4-depleted cells and consequently, SNARE pairing. Cog4-depleted HeLa cells were transiently transfected with either the wild-type or the E53/71A double mutant of Myc-tagged Cog4 containing the silent mutations within the RNAi targeting sequence. Two days later, the cells were fixed and double-immunostained with anti-Myc and either anti-GS15 or anti-GS28 antibodies. Transfected cells are indicated by arrowheads. Scale bar, 10 μm.
Figure 6
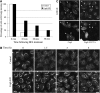
Cog4 restores Golgi-to-ER retrograde transport in a Sly1-interaction-dependent manner. (A) Depletion of Cog4 attenuates the transport of mannosidase II from the Golgi to the ER in response to BFA treatment. Control and Cog4-depleted HeLa cells were treated with BFA (5 μg/ml) for the indicated times, fixed, immunostained with anti-mannosidase II antibody, and analysed by confocal microscopy. The percentage of cells in which mannosidase II was localized to the Golgi was calculated from 200 cells at each time point. Representative confocal images are shown in Supplementary Figure S5A. (B) Depletion of Cog4 attenuates retrograde transport of KDELR. Control and Cog4-depleted HeLa cells were incubated at 15°C for the indicated times, to block protein exit from the ERGIC. The cells were then fixed, immunostained with anti-KDELR antibody and analysed by confocal microscopy. Shown are representative confocal images of control and Cog4-depleted cells at the indicated time points. Scale bar, 10 μm. (C) Cog4-depleted HeLa cells were transiently transfected with either the wild-type or E53/71A double mutant of Myc-tagged Cog4 containing the silent mutations within the RNAi targeting sequence. Two days later, the cells were incubated for 1.5 h at 15°C, fixed and double-immunostained with anti-Myc and anti-KDELR antibodies. Transfected cells are indicated by arrowheads. As shown, the wild-type Cog4, but not the E53/71A double mutant, restored retrograde transport of KDELR. Scale bar, 10 μm.
Similar articles
- Multiple SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport.
Peng R, Gallwitz D. Peng R, et al. EMBO J. 2004 Oct 13;23(20):3939-49. doi: 10.1038/sj.emboj.7600410. Epub 2004 Sep 16. EMBO J. 2004. PMID: 15372079 Free PMC article. - Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif.
Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Südhof TC. Yamaguchi T, et al. Dev Cell. 2002 Mar;2(3):295-305. doi: 10.1016/s1534-5807(02)00125-9. Dev Cell. 2002. PMID: 11879635 - SM-protein-controlled ER-associated degradation discriminates between different SNAREs.
Braun S, Jentsch S. Braun S, et al. EMBO Rep. 2007 Dec;8(12):1176-82. doi: 10.1038/sj.embor.7401105. Epub 2007 Nov 9. EMBO Rep. 2007. PMID: 18007658 Free PMC article. - Stx5-Mediated ER-Golgi Transport in Mammals and Yeast.
Linders PT, Horst CV, Beest MT, van den Bogaart G. Linders PT, et al. Cells. 2019 Jul 26;8(8):780. doi: 10.3390/cells8080780. Cells. 2019. PMID: 31357511 Free PMC article. Review. - Transport according to GARP: receiving retrograde cargo at the trans-Golgi network.
Bonifacino JS, Hierro A. Bonifacino JS, et al. Trends Cell Biol. 2011 Mar;21(3):159-67. doi: 10.1016/j.tcb.2010.11.003. Epub 2010 Dec 21. Trends Cell Biol. 2011. PMID: 21183348 Free PMC article. Review.
Cited by
- SLY1 and Syntaxin 18 specify a distinct pathway for procollagen VII export from the endoplasmic reticulum.
Nogueira C, Erlmann P, Villeneuve J, Santos AJ, Martínez-Alonso E, Martínez-Menárguez JÁ, Malhotra V. Nogueira C, et al. Elife. 2014 May 19;3:e02784. doi: 10.7554/eLife.02784. Elife. 2014. PMID: 24842878 Free PMC article. - Identifying novel genes for amyotrophic lateral sclerosis by integrating human brain proteomes with genome-wide association data.
Gu XJ, Su WM, Dou M, Jiang Z, Duan QQ, Wang H, Ren YL, Cao B, Wang Y, Chen YP. Gu XJ, et al. J Neurol. 2023 Aug;270(8):4013-4023. doi: 10.1007/s00415-023-11757-4. Epub 2023 May 6. J Neurol. 2023. PMID: 37148340 - Cog5-Cog7 crystal structure reveals interactions essential for the function of a multisubunit tethering complex.
Ha JY, Pokrovskaya ID, Climer LK, Shimamura GR, Kudlyk T, Jeffrey PD, Lupashin VV, Hughson FM. Ha JY, et al. Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15762-7. doi: 10.1073/pnas.1414829111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331899 Free PMC article. - Deep learning-based assessment of missense variants in the COG4 gene presented with bilateral congenital cataract.
Xiao B, Zhang S, Ainiwaer M, Liu H, Ning L, Hong Y, Sun Y, Ji Y. Xiao B, et al. BMJ Open Ophthalmol. 2025 Jan 14;10(1):e001906. doi: 10.1136/bmjophth-2024-001906. BMJ Open Ophthalmol. 2025. PMID: 39809522 Free PMC article. - Golgi inCOGnito: From vesicle tethering to human disease.
D'Souza Z, Taher FS, Lupashin VV. D'Souza Z, et al. Biochim Biophys Acta Gen Subj. 2020 Nov;1864(11):129694. doi: 10.1016/j.bbagen.2020.129694. Epub 2020 Jul 27. Biochim Biophys Acta Gen Subj. 2020. PMID: 32730773 Free PMC article. Review.
References
- Amarilio R, Ramachandran S, Sabanay H, Lev S (2005) Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280: 5934–5944 - PubMed
- Arac D, Dulubova I, Pei J, Huryeva I, Grishin NV, Rizo J (2005) Three-dimensional structure of the rSly1 N-terminal domain reveals a conformational change induced by binding to syntaxin 5. J Mol Biol 346: 589–601 - PubMed
- Cai H, Reinisch K, Ferro-Novick S (2007) Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 12: 671–682 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases