SARS vaccines: where are we? - PubMed (original) (raw)
Review
SARS vaccines: where are we?
Rachel L Roper et al. Expert Rev Vaccines. 2009 Jul.
Abstract
In this review, the current state of vaccine development against human severe acute respiratory syndrome (SARS) coronavirus, focusing on recently published data is assessed. We discuss which strategies have been assessed immunologically and which have been evaluated in SARS coronavirus challenge models. We discuss inactivated vaccines, virally and bacterially vectored vaccines, recombinant protein and DNA vaccines, as well as the use of attenuated vaccines. Data regarding the correlates of protection, animal models and the available evidence regarding potential vaccine enhancement of SARS disease are discussed. While there is much evidence that various vaccine strategies against SARS are safe and immunogenic, vaccinated animals still display significant disease upon challenge. Current data suggest that intranasal vaccination may be crucial and that new or combination strategies may be required for good protective efficacy against SARS in humans.
Figures
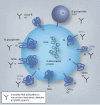
Figure 1.. Severe acute respiratory syndrome-associated coronavirus virion.
Known structural surface proteins including the viral attachment protein S, small E and M protein are shown in black text, as well as potential (gray text) predicted membrane proteins that may or may not be in the virion. Predicted orientations of the protein are also shown relative to their N, as well as the topology regarding the size of the protein mass relative to the membrane of the virus particle [1]. Nomenclature is shown as in Genbank, except for ORF14, which is designated as in the original severe acute respiratory syndrome (SARS) genome sequence manuscript [1]. For proteins that are primarily external, the number of predicted amino acids displayed on the surface of the virion is shown. Y indicates that antibodies to thiS protein have been detected in SARS patient sera [35]. E: Envelope; M: Membrane/matrix; N: Amino terminal; S: Spike.
Similar articles
- Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines.
See RH, Petric M, Lawrence DJ, Mok CPY, Rowe T, Zitzow LA, Karunakaran KP, Voss TG, Brunham RC, Gauldie J, Finlay BB, Roper RL. See RH, et al. J Gen Virol. 2008 Sep;89(Pt 9):2136-2146. doi: 10.1099/vir.0.2008/001891-0. J Gen Virol. 2008. PMID: 18753223 - [Recent developments in SARS vaccine studies].
Ruh E. Ruh E. Mikrobiyol Bul. 2010 Jul;44(3):505-17. Mikrobiyol Bul. 2010. PMID: 21064002 Review. Turkish. - Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity.
Du L, Zhao G, Chan CC, Sun S, Chen M, Liu Z, Guo H, He Y, Zhou Y, Zheng BJ, Jiang S. Du L, et al. Virology. 2009 Oct 10;393(1):144-50. doi: 10.1016/j.virol.2009.07.018. Epub 2009 Aug 15. Virology. 2009. PMID: 19683779 Free PMC article. - Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.
Cavanagh D. Cavanagh D. Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review. - Severe acute respiratory syndrome: vaccine on the way.
Zhang DM, Wang GL, Lu JH. Zhang DM, et al. Chin Med J (Engl). 2005 Sep 5;118(17):1468-76. Chin Med J (Engl). 2005. PMID: 16157050 Review. No abstract available.
Cited by
- COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines.
Iqbal Yatoo M, Hamid Z, Parray OR, Wani AH, Ul Haq A, Saxena A, Patel SK, Pathak M, Tiwari R, Malik YS, Sah R, Rabaan AA, Rodriguez Morales AJ, Dhama K. Iqbal Yatoo M, et al. Hum Vaccin Immunother. 2020 Dec 1;16(12):2891-2904. doi: 10.1080/21645515.2020.1788310. Epub 2020 Jul 23. Hum Vaccin Immunother. 2020. PMID: 32703064 Free PMC article. Review. - Development of a SARS Coronavirus Vaccine from Recombinant Spike Protein Plus Delta Inulin Adjuvant.
McPherson C, Chubet R, Holtz K, Honda-Okubo Y, Barnard D, Cox M, Petrovsky N. McPherson C, et al. Methods Mol Biol. 2016;1403:269-84. doi: 10.1007/978-1-4939-3387-7_14. Methods Mol Biol. 2016. PMID: 27076136 Free PMC article. - SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter this emerging pandemic.
Rabaan AA, Al-Ahmed SH, Sah R, Tiwari R, Yatoo MI, Patel SK, Pathak M, Malik YS, Dhama K, Singh KP, Bonilla-Aldana DK, Haque S, Martinez-Pulgarin DF, Rodriguez-Morales AJ, Leblebicioglu H. Rabaan AA, et al. Ann Clin Microbiol Antimicrob. 2020 Sep 2;19(1):40. doi: 10.1186/s12941-020-00384-w. Ann Clin Microbiol Antimicrob. 2020. PMID: 32878641 Free PMC article. Review. - Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine.
Sharma A, Virmani T, Pathak V, Sharma A, Pathak K, Kumar G, Pathak D. Sharma A, et al. Biomed Res Int. 2022 Jul 6;2022:7205241. doi: 10.1155/2022/7205241. eCollection 2022. Biomed Res Int. 2022. PMID: 35845955 Free PMC article. Review. - Mouse-adapted SARS-CoV-2 protects animals from lethal SARS-CoV challenge.
Muruato A, Vu MN, Johnson BA, Davis-Gardner ME, Vanderheiden A, Lokugamage K, Schindewolf C, Crocquet-Valdes PA, Langsjoen RM, Plante JA, Plante KS, Weaver SC, Debbink K, Routh AL, Walker D, Suthar MS, Shi PY, Xie X, Menachery VD. Muruato A, et al. PLoS Biol. 2021 Nov 4;19(11):e3001284. doi: 10.1371/journal.pbio.3001284. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34735434 Free PMC article.
References
- Marra MA, Jones SJ, Astell CR et al. The genome sequence of the SARS-associated coronavirus. Science 300(5624), 1399–1404 (2003). - PubMed
- Rota PA, Oberste MS, Monroe SS et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300(5624), 1394–1399 (2003). - PubMed
Websites
- WHO. Annex Table 2. Deaths by cause, sex and mortality stratum www.who.int/whr/2004/en/09_annexes_en.pdf
- WHO. Update 4: review of probable and laboratory-confirmed SARS cases in southern China. Epidemic and pandemic alert and response (2004) www.who.int/csr/don/2004_2001_2027/en/
- WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 26 September 2003. www.who.int/csr/sars/country/table2003_09_23/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous