Estrogen treatment in multiple sclerosis - PubMed (original) (raw)
Estrogen treatment in multiple sclerosis
Stefan M Gold et al. J Neurol Sci. 2009.
Abstract
Currently available treatments for multiple sclerosis (MS) reduce inflammatory lesions on MRI and decrease clinical relapses but have limited effects on disability. Novel treatment options that target both the inflammatory as well as the neurodegenerative component of the disease are therefore needed. A growing body of evidence from basic science and clinical studies supports the therapeutic potential of estrogens in MS. Mechanisms of action include both immunomodulatory and directly neuroprotective pathways. A first pilot trial of oral estriol treatment showed encouraging results. There are now several phase II trials underway to further determine the efficacy of estrogen treatment in MS.
Figures
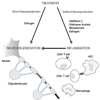
Figure 1
Direct and indirect neuroprotection in multiple sclerosis. Drugs currently approved for treatment of relapsing-remitting MS (Interferon-β, Glatiramer Acetate, Mitoxantrone) are targeted at the immune system and mainly confer anti-inflammatory effects. While this may exert some indirect neuroprotection by reducing the immune attack on neurons and oligodendrocytes, no approved treatment directly protects CNS cells and/or promotes regeneration and repair (direct neuroprotection). Studies using in vitro systems and in vivo models indicate that estrogen treatment has the potential to be both anti-inflammatory and directly neuroprotective (see text for details).
Similar articles
- Estrogen and testosterone therapies in multiple sclerosis.
Gold SM, Voskuhl RR. Gold SM, et al. Prog Brain Res. 2009;175:239-51. doi: 10.1016/S0079-6123(09)17516-7. Prog Brain Res. 2009. PMID: 19660660 Free PMC article. Review. - MS as a gateway disease.
Lublin FD. Lublin FD. J Neurol Sci. 2013 Oct 15;333(1-2):73-5. doi: 10.1016/j.jns.2013.02.014. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578792 - Multiple sclerosis at menopause: Potential neuroprotective effects of estrogen.
Christianson MS, Mensah VA, Shen W. Christianson MS, et al. Maturitas. 2015 Feb;80(2):133-9. doi: 10.1016/j.maturitas.2014.11.013. Epub 2014 Nov 27. Maturitas. 2015. PMID: 25544310 Review. - Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice.
Tiwari-Woodruff S, Voskuhl RR. Tiwari-Woodruff S, et al. J Neurol Sci. 2009 Nov 15;286(1-2):81-5. doi: 10.1016/j.jns.2009.04.023. Epub 2009 May 13. J Neurol Sci. 2009. PMID: 19442988 Free PMC article. - Review of laquinimod and its therapeutic potential in multiple sclerosis.
Thöne J, Gold R. Thöne J, et al. Expert Opin Pharmacother. 2013 Dec;14(18):2545-52. doi: 10.1517/14656566.2013.848855. Epub 2013 Nov 11. Expert Opin Pharmacother. 2013. PMID: 24215556 Review.
Cited by
- Males still dominate animal studies.
Zucker I, Beery AK. Zucker I, et al. Nature. 2010 Jun 10;465(7299):690. doi: 10.1038/465690a. Nature. 2010. PMID: 20535186 No abstract available. - Nestorone® , a 19nor-progesterone derivative boosts remyelination in an animal model of demyelination.
El-Etr M, Akwa Y, Rame M, Schumacher M, Sitruk-Ware R. El-Etr M, et al. CNS Neurosci Ther. 2021 Apr;27(4):464-469. doi: 10.1111/cns.13538. Epub 2020 Dec 24. CNS Neurosci Ther. 2021. PMID: 33369182 Free PMC article. - Disease Progression and Obstetric Outcomes of Women with Multiple Sclerosis at a Reference Center in Northeastern Brazil.
Barros GMC, Oliveira BES, Oliveira GJ, Silva RKP, Cardoso TN, Maia SB. Barros GMC, et al. Rev Bras Ginecol Obstet. 2021 Mar;43(3):165-171. doi: 10.1055/s-0040-1722157. Epub 2021 Apr 15. Rev Bras Ginecol Obstet. 2021. PMID: 33860499 Free PMC article. - Practical Evidence-Based Recommendations for Patients with Multiple Sclerosis Who Want to Have Children.
Fragoso YD, Adoni T, Brooks JBB, Finkelsztejn A, da Gama PD, Grzesiuk AK, Marques VD, Parolin MFK, Sato HK, Varela DL, Vasconcelos CCF. Fragoso YD, et al. Neurol Ther. 2018 Dec;7(2):207-232. doi: 10.1007/s40120-018-0110-3. Epub 2018 Aug 30. Neurol Ther. 2018. PMID: 30167914 Free PMC article. Review. - Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential.
Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Paterni I, et al. Steroids. 2014 Nov;90:13-29. doi: 10.1016/j.steroids.2014.06.012. Epub 2014 Jun 24. Steroids. 2014. PMID: 24971815 Free PMC article. Review.
References
- Brex PA, Jenkins R, Fox NC, Crum WR, O'Riordan JI, Plant GT, et al. Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology. 2000 Apr 25;54(8):1689–1691. - PubMed
- Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003 Feb;126(Pt 2):433–437. - PubMed
- Ge Y, Grossman RI, Udupa JK, Wei L, Mannon LJ, Polansky M, et al. Brain atrophy in relapsing-remitting multiple sclerosis and secondary progressive multiple sclerosis: longitudinal quantitative analysis. Radiology. 2000;214(3):665–670. - PubMed
- Losseff NA, Wang L, Lai HM, Yoo DS, Gawne CM, McDonald WI, et al. Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain. 1996:2009–2019. - PubMed
- Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53(8):1698–1704. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS045443-04/NS/NINDS NIH HHS/United States
- K24 NS062117/NS/NINDS NIH HHS/United States
- R01 AI050839-08/AI/NIAID NIH HHS/United States
- R01 AI050839/AI/NIAID NIH HHS/United States
- K24 NS062117-01/NS/NINDS NIH HHS/United States
- R01 NS051591-03/NS/NINDS NIH HHS/United States
- R01 NS045443/NS/NINDS NIH HHS/United States
- R01 NS051591/NS/NINDS NIH HHS/United States
- R01 AI050839-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical