The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF - PubMed (original) (raw)
The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF
Sean M Anderson et al. Biomaterials. 2009 Sep.
Abstract
Growth factors are a class of signaling proteins that direct cell fate through interaction with cell-surface receptors. Although a myriad of possible cell fates stems from a growth factor binding to its receptor, the signaling cascades that result in one fate over another are still being elucidated. One possible mechanism by which nature modulates growth factor signaling is through the method of presentation of the growth factor--soluble or immobilized (matrix bound). Here we present the methodology to study signaling of soluble versus immobilized VEGF through VEGFR-2. We have designed a strategy to covalently immobilize VEGF using its heparin-binding domain to orient the molecule (bind) and a secondary functional group to mediate covalent binding (lock). This bind-and-lock approach aims to allow VEGF to assume a bioactive orientation before covalent immobilization. Surface plasmon resonance (SPR) demonstrated heparin and VEGF binding with surface densities of 60 ng/cm2 and 100 pg/cm2, respectively. ELISA experiments confirmed VEGF surface density and showed that electrostatically bound VEGF releases in cell medium and heparin solutions while covalently bound VEGF remains immobilized. Electrostatically bound VEGF and covalently bound VEGF phosphorylate VEGFR-2 in both VEGFR-2 transfected cells and VEGFR-2 endogenously producing cells. HUVECs plated on VEGF functionalized surfaces showed different morphologies between surface-bound VEGF and soluble VEGF. The surfaces synthesized in these studies allow for the study of VEGF/VEGFR-2 signaling induced by covalently bound, electrostatically bound, and soluble VEGF and may provide further insight into the design of materials for the generation of a mature and stable vasculature.
Figures
Figure 1
Cartoon of our bind-and-lock immobilization strategy for VEGF. VEGF is incubated with a heparin-modified surface and allowed to electrostatically bind through the heparin-binding domain of VEGF (A). For covalent immobilization the heparin modified surface also contains a photoreactive group, p-azidobenzoyl, that can be activated post VEGF binding (B). Thus, this approach uses specific electrostatic (non-covalent) interactions first to orient the growth factor through its heparin binding domain (bind) and then a covalent bond to keep it in place (lock).
Figure 2
Infrared spectra of unmodified heparin (solid line) and oxidized heparin (dotted line). The oxidation of heparin was tracked by appearance of a sharp peak at 1730 cm−1(arrow).
Figure 3
(A) Ellipsometry plot of unmodified gold and EG-NH2 modified gold before and after UV treatment. (B) Ellipsometry plot of unmodified gold and EG-OH modified gold before and after piranha clean. Both EG-NH2 and EG-OH modification was tracked through the positive displacement in the ellipsometry plot. UV treatment did not affect the EG-NH2 SAM, while piranha clean removes the EG-OH SAM and etches the surface.
Figure 4
Surface plasmon resonance (SPR) for oxidized heparin immobilization to EG-NH2 and EG-OH functionalized surface. SPR steps are: (A) Heparin injection (1 mg/ml, 30 min), (B) Sodium cyanoborohydride injection (100 mM, 7 min), (C) Tris injection (100 mM, 6 min), and (D) Tween washes (0.05% in PBS, 5 min/each). Data indicated that heparin is stably bound at about 60 ng/cm2. All injections were done at 5 µl/min and PBS was used between injections to wash and stabilize the baseline. Following all the injections, oxidized heparin was only immobilized to the surface that contained amines, indicating that 1% EG-NH2 surface is sufficient for heparin immobilization.
Figure 5
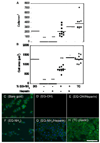
Cell attachment and spreading on heparin-modified surfaces shows ability to adhere fibronectin and support cell growth. (A) Cell attachment on bare gold (BG), SAMs functionalized with EG-OH, EG-OH/heparin, EG-NH2 and EG-NH2/heparin, and tissue culture plastic (TC). (B) Cell spreading on bare gold, SAMs functionalized with EG-OH, EG-OH/heparin, EG-NH2 and EG-NH2/heparin, and tissue culture plastic (TC). Cells were stained for actin (green) and nucleus (blue) for cells seeded on (C) bare gold surface and SAMs functionalized with (D) EG-OH, (E) EG-OH/heparin, (F) EG-NH2, and (G) EG-NH2/heparin. As an additional control, cells were seeded on tissue culture plastic (H). Scale bar is 100 µm. The symbol *** indicates statistical significance to a level of p < 0.001 using one-way ANOVA with a Tukey post-hoc test, which compares all possible pairs.
Figure 6
Surface plasmon resonance (SPR) data of VEGF binding to a heparin or 1% EG-NH2 surface. SPR steps are: (A) Heparin (1 mg/ml) or PBS injection (30 min), (B) Sodium cyanoborohydride injection (100 mM, 7 min), (C) Tris injection (100 mM, 6 min), (D) Tween washes (0.05% in PBS, 5 min/each), and (E) VEGF injection (200 ng/ml, 15 min). The bottom plot represents a close up view of the dotted square in the top plot, which shows the VEGF binding step. VEGF did not bind to the 1% EG-NH2 surface in the absence of heparin, with the baseline returning to the initial value. For surfaces that contained heparin, VEGF was bound at 100 pg/cm2. All injections were done at 5 µl/min and PBS was used between injections to wash and stabilize the baseline.
Figure 7
ELISA readings and release rates for VEGF immobilized to 1% EG-NH2, 1% EG-NH2/Heparin-ABH (covalent), and 1% EG-NH2/Heparin (electrostatic) surfaces. (A) Release curves of VEGF showed that VEGF was stably bound to heparin-functionalized surfaces, but not to 1% EG-NH2 surfaces. (B) Direct ELISA measurements of VEGF on modified surfaces after release confirmed findings from SPR, with covalent and electrostatically bound VEGF immobilized at a density of 100 pg/cm2. (C) Release curves for electrostatically bound and covalently bound VEGF in heparin (H, 1 mg/ml heparin) and PAE/KDR conditioned media (CM) show significantly more release for electrostatically bound VEGF than for covalently bound VEGF (p < 0.05). (D) Direct ELISA measurements of VEGF on surface after release confirmed that covalently bound VEGF remained on surface while electrostatically bound VEGF released. The symbol * indicates statistical significance to a level of p < 0.05 using one-way ANOVA with a Tukey post-hoc test, which compares all possible pairs.
Figure 8
Western blot analysis of PAE/KDR cells (A) and HUVECs (B) brought into contact with VEGF modified surfaces or exposed to soluble VEGF. (A) Top band shows phosphorylated VEGFR-2 (pVEGFR-2), while bottom band shows total VEGFR-2. Plot quantifies phosphorylated VEGFR-2 band intensities and is normalized to total VEGFR-2 for each condition (n = 5 blots). Surface bound VEGF phosphorylates VEGFR-2 to a greater extent than background in the negative control. Soluble VEGF does not significantly increase phosphorylation signal over surface bound VEGF. (B) Top band shows pVEGFR-2 and bottom band shows total VEGFR-2. Plot quantifies phosphorylated VEGFR-2 band intensities and is normalized to total VEGFR-2 for each condition (n = 3 blots, representative blot shown). Both electrostatically and covalently bound VEGF were able to phosphorylate VEGFR-2 in PAE/KDR cells and HUVECs. The symbol * indicates statistical significance to a level of p < 0.05 using one-way ANOVA with a Tukey post-hoc test, which compares all possible pairs.
Figure 9
Cell proliferation of HUVECs on VEGF-modified surfaces confirms VEGF mitogen activity in immobilized state. At day 5, the cell number on VEGF-modified surfaces is significantly greater than the cell count on the negative control. The symbol *, **, and *** indicate statistical significance to a level of p < 0.05, p < 0.01, and p < 0.001, respectively, using one-way ANOVA with a Tukey post-hoc test, which compares all possible pairs.
Figure 10
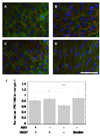
Fluorescent microscopy of HUVECs on VEGF modified surfaces. Cells were stained for the nuclei (blue), actin (green) and PECAM-1 (CD31, red). HUVECs were plated on (A) covalently bound VEGF and (B) electrostatically bound VEGF surfaces. As controls, HUVECs were plated on surfaces that contained heparin and fibronectin but no bound VEGF (C, negative control) and surfaces that were treated with soluble VEGF (200 ng/ml, positive control, D). Soluble VEGF treatment resulted in different morphology from the other conditions with cells stretched and polarized in a single direction (D). (E) Quantification of PECAM intensity showed an increase in intensity for surface bound and soluble VEGF over negative control. Scale bar is 100 µm. The symbols * and *** indicate statistical significance to a level of p < 0.05 and p < 0.001 using one-way ANOVA with a Tukey post-hoc test, which compares all possible pairs.
Similar articles
- VEGF internalization is not required for VEGFR-2 phosphorylation in bioengineered surfaces with covalently linked VEGF.
Anderson SM, Shergill B, Barry ZT, Manousiouthakis E, Chen TT, Botvinick E, Platt MO, Iruela-Arispe ML, Segura T. Anderson SM, et al. Integr Biol (Camb). 2011 Sep;3(9):887-96. doi: 10.1039/c1ib00037c. Epub 2011 Aug 8. Integr Biol (Camb). 2011. PMID: 21826315 Free PMC article. - Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering.
Chiu LL, Weisel RD, Li RK, Radisic M. Chiu LL, et al. J Tissue Eng Regen Med. 2011 Jan;5(1):69-84. doi: 10.1002/term.292. Epub 2010 Aug 17. J Tissue Eng Regen Med. 2011. PMID: 20717888 - The promotion of endothelial cell attachment and spreading using FNIII10 fused to VEGF-A165.
Traub S, Morgner J, Martino MM, Höning S, Swartz MA, Wickström SA, Hubbell JA, Eming SA. Traub S, et al. Biomaterials. 2013 Aug;34(24):5958-68. doi: 10.1016/j.biomaterials.2013.04.050. Epub 2013 May 14. Biomaterials. 2013. PMID: 23683723 - Low molecular weight fucoidan increases VEGF165-induced endothelial cell migration by enhancing VEGF165 binding to VEGFR-2 and NRP1.
Lake AC, Vassy R, Di Benedetto M, Lavigne D, Le Visage C, Perret GY, Letourneur D. Lake AC, et al. J Biol Chem. 2006 Dec 8;281(49):37844-52. doi: 10.1074/jbc.M600686200. Epub 2006 Oct 6. J Biol Chem. 2006. PMID: 17028197 - Synergistic Binding of Vascular Endothelial Growth Factor-A and Its Receptors to Heparin Selectively Modulates Complex Affinity.
Teran M, Nugent MA. Teran M, et al. J Biol Chem. 2015 Jun 26;290(26):16451-62. doi: 10.1074/jbc.M114.627372. Epub 2015 May 15. J Biol Chem. 2015. PMID: 25979342 Free PMC article.
Cited by
- Sequential sequestrations increase the incorporation and retention of multiple growth factors in mineralized collagen scaffolds.
Tiffany AS, Dewey MJ, Harley BAC. Tiffany AS, et al. RSC Adv. 2020;10(45):26982-26996. doi: 10.1039/d0ra03872e. Epub 2020 Jul 20. RSC Adv. 2020. PMID: 33767853 Free PMC article. - Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis.
Shepard JA, Virani FR, Goodman AG, Gossett TD, Shin S, Shea LD. Shepard JA, et al. Biomaterials. 2012 Oct;33(30):7412-21. doi: 10.1016/j.biomaterials.2012.06.081. Epub 2012 Jul 15. Biomaterials. 2012. PMID: 22800542 Free PMC article. - Oncotically Driven Control over Glycocalyx Dimension for Cell Surface Engineering and Protein Binding in the Longitudinal Direction.
Siren EMJ, Chapanian R, Constantinescu I, Brooks DE, Kizhakkedathu JN. Siren EMJ, et al. Sci Rep. 2018 May 15;8(1):7581. doi: 10.1038/s41598-018-25870-2. Sci Rep. 2018. PMID: 29765073 Free PMC article. - Synthetic microparticles conjugated with VEGF165 improve the survival of endothelial progenitor cells via microRNA-17 inhibition.
Aday S, Zoldan J, Besnier M, Carreto L, Saif J, Fernandes R, Santos T, Bernardino L, Langer R, Emanueli C, Ferreira L. Aday S, et al. Nat Commun. 2017 Sep 29;8(1):747. doi: 10.1038/s41467-017-00746-7. Nat Commun. 2017. PMID: 28963481 Free PMC article. - Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke.
Nih LR, Gojgini S, Carmichael ST, Segura T. Nih LR, et al. Nat Mater. 2018 Jul;17(7):642-651. doi: 10.1038/s41563-018-0083-8. Epub 2018 May 21. Nat Mater. 2018. PMID: 29784996 Free PMC article.
References
- Ehrbar M, Zeisberger SM, Raeber GP, Hubbell JA, Schnell C, Zisch AH. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29(11):1720–1729. - PubMed
- Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115(3):353–360. - PubMed
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. - PubMed
- Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure. 1998;6(5):637–648. - PubMed
- Catena R, Muniz-Medina V, Moralejo B, Javierre B, Best CJ, Emmert-Buck MR, et al. Increased expression of VEGF121/VEGF165-189 ratio results in a significant enhancement of human prostate tumor angiogenesis. Int J Cancer. 2007;120(10):2096–2109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources